The Global Decline in Human Fertility: The Post-Transition Trap Hypothesis
Abstract
:1. Introduction
2. Defining Terms—Measures of Fertility
3. Pre-Transition Phase of Human Evolution
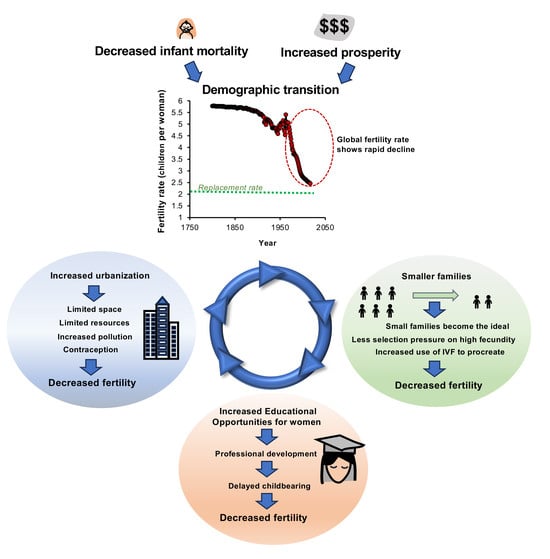
4. Fertility and the Demographic Transition
5. Mechanisms Responsible for TFR Decline
6. Transition to Sub-Replacement Fertility
7. Post-Transition Societies
7.1. Sub-Replacement Fertility Levels Are Difficult to Reverse
7.2. The Cultural Forces That Keep Fertility Levels Low—The Power of One
8. Long-Term Consequences of Sub-Replacement TFR
9. Role of the Assisted-Conception Industry
10. Is Fecundity Changing?
11. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Aitken, R.J. The Infertility Trap; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Aitken, R.J. The changing tide of human fertility. Hum. Reprod. 2022, 37, 629–638. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects. 2022. Available online: https://population.un.org/wpp/ (accessed on 1 September 2023).
- Bryant, J. Theories of fertility decline and the evidence from development indicators. Popul. Dev. Rev. 2007, 33, 101–127. [Google Scholar] [CrossRef]
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Gietel-Basten, S.; Sobotka, T. Trends in population health and demography. Lancet 2021, 398, 580–581. [Google Scholar] [CrossRef] [PubMed]
- Keilman, N. Trends in population health and demography. Lancet 2021, 398, 581. [Google Scholar] [CrossRef] [PubMed]
- Maaløe, N.; Housseine, N.; Meguid, T.; Tellier, S.; van Roosmalen, J.; Meyrowitsch, D.W.; van den Akker, T. Trends in population health and demography. Lancet 2021, 398, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Diez Medrano, J. Results, dilemmas, and suggestions concerning the demographic transition theory: Causes of the decline of fertility in the nineteenth century. Bol. Asoc. Demogr. Hist. 1985, 3, 4–20. [Google Scholar]
- Hirschman, C. Why fertility changes. Annu. Rev. Sociol. 1994, 20, 203–233. [Google Scholar] [CrossRef]
- Canning, D. The causes and consequences of demographic transition. Popul. Stud. 2011, 65, 353–361. [Google Scholar] [CrossRef]
- Galor, O. The demographic transition: Causes and consequences. Cliometrica 2012, 6, 1–28. [Google Scholar] [CrossRef]
- Burger, O.; DeLong, J.P. What if fertility decline is not permanent? The need for an evolutionarily informed approach to understanding low fertility. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150157. [Google Scholar] [CrossRef]
- Wilson, C. Thinking about post-transitional demographic regimes: A reflection. Demogr. Res. 2013, 28, 1373–1388. [Google Scholar] [CrossRef]
- te Velde, E.; Burdorf, A.; Nieschlag, E.; Eijkemans, R.; Kremer, J.A.M.; Roeleveld, N.; Habbema, D. Is human fecundity declining in Western countries? Hum. Reprod. 2010, 25, 1348–1353. [Google Scholar] [CrossRef]
- Penn, D. Explaining the human demographic transition. Trends Ecol. Evol. 1999, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.O.; Russell, A.F.; Lummaa, V. When fecundity does not equal fitness: Evidence of an offspring quantity versus quality trade-off in pre-industrial humans. Proc. R. Soc. B Biol. Sci. 2008, 275, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Bongaarts, J.; Feeney, G. On the quantum and tempo of fertility. Popul. Dev. Rev. 1998, 1, 271–291. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, Q.; Sánchez-Barricarte, J.J. China’s fertility change: An analysis with multiple measures. Popul. Health Metr. 2022, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Schoen, R. Relating period and cohort fertility. Demography 2022, 59, 877–894. [Google Scholar] [CrossRef]
- World Bank Open Data. 2023. Available online: https://data.worldbank.org/indicator/SP.DYN.TFRT.IN (accessed on 14 December 2023).
- Human Fertility Database. Max Planck Institute for Demographic Research (Germany) and Vienna Institute of Demography (Austria). Available online: https://www.humanfertility.org (accessed on 14 December 2023).
- Bongaarts, J. A method for the estimation of fecundability. Demography 1975, 12, 645–660. [Google Scholar] [CrossRef]
- Zinaman, M.J.; Clegg, E.D.; Brown, C.C.; O’Connor, J.; Selevan, S.G. Estimates of human fertility and pregnancy loss. Fertil. Steril. 1996, 65, 503–509. [Google Scholar] [CrossRef]
- Joffe, M. What has happened to human fertility? Hum. Reprod. 2010, 25, 295–307. [Google Scholar] [CrossRef]
- Gaillard, J.-M.; Festa-Bianchet, M.; Yoccoz, N.G.; Loison, A.; Toigo, C. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 367–393. [Google Scholar] [CrossRef]
- Gaillard, J.-M.; Sempéré, A.J.; Boutin, J.-M.; Laere, G.V.; Boisaubert, B. Effects of age and body weight on the proportion of females breeding in a population of roe deer (Capreolus capreolus). Can. J. Zool. 1992, 70, 1541–1545. [Google Scholar] [CrossRef]
- Wilson, C. Understanding the nature and importance of low-growth demographic regimes. In Asian Historical Demography; Liu, T.J., Lee, J., Reher, D.S., Saito, O., Feng, W., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 24–44. [Google Scholar]
- Jones, J.H.; Bird, R.B. The marginal valuation of fertility. Evol. Hum. Behav. 2014, 35, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Page, A.E.; French, J.C. Reconstructing prehistoric demography: What role for extant hunter-gatherers? Evol. Anthropol. 2020, 29, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Page, A.E.; Viguier, S.; Dyble, M.; Smith, D.; Chaudhary, N.; Salali, G.D.; Thompson, J.; Vinicius, L.; Mace, R.; Migliano, A.B. Reproductive trade-offs in extant hunter-gatherers suggest adaptive mechanism for the Neolithic expansion. Proc. Natl. Acad. Sci. USA 2016, 113, 4694–4699. [Google Scholar] [CrossRef]
- Eaton, J.W.; Mayer, A.J. The social biology of very high fertility among the Hutterites: The demography of a unique population. Hum. Biol. 1953, 25, 206–264. [Google Scholar]
- Konner, M. Nursing frequency and birth spacing in Kung hunter-gatherers. IPPF Med. Bull. 1978, 15, 1–3. [Google Scholar]
- Volk, A.A.; Atkinson, J.A. Infant and child death in the human environment of evolutionary adaptation. Evol. Hum. Behav. 2013, 34, 182–192. [Google Scholar] [CrossRef]
- Malthus, T. An essay on the principle of population 1798. In The Works of Thomas Robert Malthus; Pickering & Chatto Publishers: London, UK, 1986; pp. 1–39. [Google Scholar]
- Khan, A. The industrial revolution and the demographic transition. Bus. Rev. 2008, 1, 9–15. [Google Scholar]
- Griffin, E. A Short History of the British Industrial Revolution; Bloomsbury Publishing: London, UK, 2018. [Google Scholar]
- Chu, A.C.; Peretto, P.F.; Wang, X. Agricultural revolution and industrialization. J. Dev. Econ. 2022, 158, 102887. [Google Scholar] [CrossRef]
- Peng, X. Demographic consequences of the Great Leap Forward in China’s provinces. Popul. Dev. Rev. 1987, 13, 639–670. [Google Scholar] [CrossRef]
- Sato, Y.; Yamamoto, K. Population concentration, urbanization, and demographic transition. J. Urban Econ. 2005, 58, 45–61. [Google Scholar] [CrossRef]
- Reher, D.S. The demographic transition revisited as a global process. Popul. Space Place 2004, 10, 19–41. [Google Scholar] [CrossRef]
- Reher, D. Back to the basics: Mortality and fertility interactions during the demographic transition. Contin. Change 1999, 14, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J. The effects of improved survival on fertility: A reassessment. Popul. Dev. Rev. 2001, 27, 60–92. [Google Scholar]
- Lutz, W.; Skirbekk, V.; Testa, M.R. The low-fertility trap hypothesis: Forces that may lead to further postponement and fewer births in Europe. Vienna Yearb. Popul. Res. 2006, 4, 167–192. [Google Scholar] [CrossRef]
- Mussino, E.; Ortensi, L.E. The same fertility ideals as in the country of origin? A study of the personal ideal family size among immigrant women in Italy. Comp. Popul. Stud. 2018, 43, 243–274. [Google Scholar] [CrossRef]
- Zhong, J.; Gao, J.; Liu, C.; Huang, J.; Luo, R. Quantity–quality trade-off and early childhood development in rural family: Evidence from China’s Guizhou province. Int. J. Environ. Res. Public Health 2019, 16, 1307. [Google Scholar] [CrossRef]
- McDonald, P. Societal foundations for explaining low fertility: Gender equity. Demogr. Res. 2013, 28, 981–994. Available online: http://www.jstor.org/stable/26349977 (accessed on 1 February 2024). [CrossRef]
- Thornton, A. The developmental paradigm, reading history sideways, and family change. Demography 2001, 38, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A. Reading History Sideways: The Fallacy and Enduring Impact of the Developmental Paradigm on Family Life; University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Thornton, A.; Binstock, G.; Yount, K.M.; Abbasi-Shavazi, M.J.; Ghimire, D.; Xie, Y. International fertility change: New data and insights from the developmental idealism framework. Demography 2012, 49, 677–698. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.; Dorius, S.F.; Swindle, J. Developmental Idealism: The cultural foundations of world development programs. Soc. Dev. 2015, 1, 277–320. [Google Scholar] [CrossRef] [PubMed]
- Eijkemans, M.J.; Van Poppel, F.; Habbema, D.F.; Smith, K.R.; Leridon, H.; te Velde, E.R. Too old to have children? Lessons from natural fertility populations. Hum. Reprod. 2014, 29, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Lesthaeghe, R. The second demographic transition: A concise overview of its development. Proc. Natl. Acad. Sci. USA 2014, 111, 18112–18115. [Google Scholar] [CrossRef]
- Pourreza, A.; Sadeghi, A.; Amini-Rarani, M.; Khodayari-Zarnaq, R.; Jafari, H. Contributing factors to the total fertility rate declining trend in the Middle East and North Africa: A systemic review. J. Health Popul. Nutr. 2021, 40, 11. [Google Scholar] [CrossRef]
- Padilla, S.L.; Garcia, J.E. Effect of maternal age and number of in vitro fertilization procedures on pregnancy outcome. Fertil. Steril. 1989, 52, 270–273. [Google Scholar] [CrossRef]
- Weston, R.; Qu, L.; Parker, R. ‘It’s Not for Lack of Wanting Kids…’ A Report on the Fertility Decision Making Project; Australian Institute of Family Studies: Melbourne, Australia, 2004. Available online: https://aifs.gov.au/research/research-reports/its-not-lack-wanting-kids-report-fertility-decision-making-project (accessed on 14 December 2023).
- Koropeckyj-Cox, T.; Pendell, G. Attitudes about childlessness in the United States: Correlates of positive, neutral, and negative responses. J. Fam. Issues 2007, 28, 1054–1082. [Google Scholar] [CrossRef]
- Noordhuizen, S.; de Graaf, P.; Sieben, I. The public acceptance of voluntary childlessness in the Netherlands: From 20 to 90 per cent in 30 years. Soc. Indic. Res. 2010, 99, 163–181. [Google Scholar] [CrossRef]
- Miettinen, A.; Szalma, I. Childlessness intentions and ideals in Europe. Finn. Yearb. Popul. Res. 2014, 49, 31–55. [Google Scholar] [CrossRef]
- Hwang, J. Later, fewer, none? Recent trends in cohort fertility in South Korea. Demography 2023, 60, 563–582. [Google Scholar] [CrossRef]
- Hofsten, E. Demographic transition and economic development in Albania. Eur. Demogr. Inf. Bull. 1975, 6, 147–158. [Google Scholar] [CrossRef]
- Mills, M.; Rindfuss, R.R.; McDonald, P.; te Velde, E.; ESHRE Reproduction and Society Task Force. Why do people postpone parenthood? Reasons and social policy incentives. Hum. Reprod. Update 2011, 17, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.J.A.; Perry, C.; Saraswati, C.M.; Judge, M.A.; Heyworth, J.; Le Souëf, P.N. Lower infant mortality, higher household size, and more access to contraception reduce fertility in low-and middle-income nations. PLoS ONE 2021, 18, e0280260. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Jaafaripooyan, E.; Vedadhir, A.A.; Foroushani, A.R.; Ahadinejad, B.; Pourreza, A. Socio-economic factors influencing on total fertility rate in Iran: A panel data analysis for the period of 2002–2012. Electron. Physician 2016, 8, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Staetsky, L.D. Stalling fertility decline of Israeli Muslims and the demographic transition theory. Popul. Stud. 2019, 73, 317–333. [Google Scholar] [CrossRef]
- Little, B.B.; Malina, R.M.; Reyes, M.E.P. Natural selection and demographic transition in a Zapotec-speaking genetic isolate in the Valley of Oaxaca, southern Mexico. Ann. Hum. Biol. 2008, 35, 34–49. [Google Scholar] [CrossRef]
- Low, B.S. Ecological demography: A synthetic focus in evolutionary anthropology. Evol. Anthropol. 1993, 1, 177–187. [Google Scholar] [CrossRef]
- Sear, R. Evolutionary contributions to the study of human fertility. Popul. Stud. 2015, 69, S39–S55. [Google Scholar] [CrossRef]
- Colleran, H. The cultural evolution of fertility decline. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150152. [Google Scholar] [CrossRef]
- Lesthaeghe, R.; van de Kaa, D. Twee demografische transities? In Bevolking–Groei en Krimp, Mens en Maatschappij; Lesthaeghe, R., van de Kaa, D., Eds.; Van Loghum-Slaterus: Deventer, The Netherlands, 1986; pp. 9–24. [Google Scholar]
- United Nations. World Population Prospects: The 1998 Revision. New York. Available online: https://press.un.org/en/1998/19981027.pop684.html (accessed on 14 December 2023).
- Myrskylä, M.; Kohler, H.-P.; Billari, F.C. Advances in development reverse fertility declines. Nature 2009, 460, 741–743. [Google Scholar] [CrossRef]
- Espenshade, T.J. Population dynamics with immigration and low fertility. Popul. Dev. Rev. 1986, 12, 248–261. [Google Scholar] [CrossRef]
- Lesthaeghe, R. The second demographic transition, 1986–2020: Sub-replacement fertility and rising cohabitation—A global update. Genus 2020, 76, 10. [Google Scholar] [CrossRef]
- Kohler, H.-P.; Billari, F.C.; Ortega, J.A. The emergence of lowest-low fertility in Europe during the 1990s. Popul. Dev. Rev. 2002, 28, 641–680. [Google Scholar] [CrossRef]
- Hellstrand, J.; Nisén, J.; Miranda, V.; Fallesen, P.; Dommermuth, L.; Myrskylä, M. Not just later, but fewer: Novel trends in cohort fertility in the Nordic countries. Demography 2021, 58, 1373–1399. [Google Scholar] [CrossRef]
- Newsham, N.; Rowe, F. Understanding trajectories of population decline across rural and urban Europe: A sequence analysis. Popul. Space Place 2023, 29, e2630. [Google Scholar] [CrossRef]
- McAuliffe, M.; Khadria, B. World Migration Report 2020; IOM: Geneva, Switzerland, 2019. [Google Scholar]
- Dascălu, D.I. Individualism and mass communication in the context of globalization. Procedia Soc. Behav. Sci. 2014, 163, 1–6. [Google Scholar] [CrossRef]
- Bolund, E.; Hayward, A.; Pettay, J.E.; Lummaa, V. Effects of the demographic transition on the genetic variances and covariances of human life-history traits. Evolution 2015, 69, 747–755. [Google Scholar] [CrossRef]
- Zorrilla, M.; Yatsenko, A.N. The genetics of infertility: Current status of the field. Curr. Genet. Med. Rep. 2013, 1, 247–260. [Google Scholar] [CrossRef]
- Capalbo, A.; Poli, M.; Riera-Escamilla, A.; Shukla, V.; Kudo Høffding, M.; Krausz, C.; Hoffmann, E.R.; Simon, C. Preconception genome medicine: Current state and future perspectives to improve infertility diagnosis and reproductive and health outcomes based on individual genomic data. Hum. Reprod. Update 2021, 27, 254–279. [Google Scholar] [CrossRef]
- Houston, B.J.; Riera-Escamilla, A.; Wyrwoll, M.J.; Salas-Huetos, A.; Xavier, M.J.; Nagirnaja, L.; Friedrich, C.; Conrad, D.F.; Aston, K.I.; Krausz, C.; et al. A systematic review of the validated monogenic causes of human male infertility: 2020 update and a discussion of emerging gene-disease relationships. Hum. Reprod. Update 2021, 28, 15–29. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Qu, R.; Zhang, W.; Tan, Y.; Sha, Y.; Li, L.; Yin, T. Genetic mechanisms of fertilization failure and early embryonic arrest: A comprehensive review. Hum. Reprod. 2024, 30, 48–80. [Google Scholar] [CrossRef]
- Mohallem, S.V.; de Araújo Lobo, D.J.; Pesquero, C.R.; Assunção, J.V.; de Andre, P.A.; Saldiva, P.H.N.; Dolhnikoff, M. Decreased fertility in mice exposed to environmental air pollution in the city of Sao Paulo. Environ. Res. 2005, 98, 196–202. [Google Scholar] [CrossRef]
- Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Levine, H.; Andersson, A.-M.; Jørgensen, N.; Main, K.M.; Lidegaard, Ø.; Priskorn, L.; Holmboe, S.A.; Bräuner, E.V.; et al. Environmental factors in declining human fertility. Nat. Rev. Endocrinol. 2022, 18, 139–157. [Google Scholar] [CrossRef]
- Xu, R.; Zhong, Y.; Li, R.; Li, Y.; Zhong, Z.; Liu, T.; Wang, Q.; Lv, Z.; Huang, S.; Duan, Y.-G.; et al. Association between exposure to ambient air pollution and semen quality: A systematic review and meta-analysis. Sci. Total Environ. 2023, 870, 161892. [Google Scholar] [CrossRef]
- Aitken, R.J. Role of sperm DNA damage in creating de-novo mutations in human offspring: The ‘post-meiotic oocyte collusion’ hypothesis. Reprod. Biomed. Online 2022, 45, 109–124. [Google Scholar] [CrossRef]
- Erenpreisa, J.; Vainshelbaum, N.M.; Lazovska, M.; Karklins, R.; Salmina, K.; Zayakin, P.; Rumnieks, F.; Inashkina, I.; Pjanova, D.; Erenpreiss, J. The price of human evolution: Cancer-testis antigens, the decline in male fertility and the increase in cancer. Int. J. Mol. Sci. 2023, 24, 11660. [Google Scholar] [CrossRef]
- Wallach, E.E.; Gindoff, P.R.; Jewelewicz, R. Reproductive potential in the older woman. Fertil. Steril. 1986, 46, 989–1001. [Google Scholar] [CrossRef]
- Mathews, T.J.; Hamilton, B.E. Mean age of mothers is on the rise: United States, 2000–2014. NCHS Data Brief 2016, 232, 1–8. [Google Scholar]
- De Geyter, C.; Wyns, C.; Calhaz-Jorge, C.; de Mouzon, J.; Ferraretti, A.P.; Kupka, M.; Nyboe Andersen, A.; Nygren, K.G.; Goossens, V. 20 years of the European IVF-monitoring Consortium registry: What have we learned? A comparison with registries from two other regions. Hum. Reprod. 2020, 35, 2832–2849. [Google Scholar] [CrossRef]
- Haddad, M.; Stewart, J.; Xie, P.; Cheung, S.; Trout, A.; Keating, D.; Parrella, A.; Lawrence, S.; Rosenwaks, Z.; Palermo, G.D. Thoughts on the popularity of ICSI. J. Assist. Reprod. Genet. 2021, 38, 101–123. [Google Scholar] [CrossRef]
- Liu, X.-H.; Qiao, J.; Li, R.; Yan, L.-Y.; Chen, L.-X. Y chromosome AZFc microdeletion may not affect the outcomes of ICSI for infertile males with fresh ejaculated sperm. J. Assist. Reprod. Genet. 2013, 30, 813–819. [Google Scholar] [CrossRef]
- Chen, D.; Fan, G.; Zhu, X.; Chen, Q.; Chen, X.; Gao, F.; Guo, Z.; Luo, P.; Gao, Y. Y chromosome microdeletions in Chinese men with infertility: Prevalence, phenotypes, and intracytoplasmic sperm injection outcomes. Reprod. Biol. Endocrinol. 2023, 21, 116. [Google Scholar] [CrossRef]
- Ferreux, L.; Bourdon, M.; Chargui, A.; Schmitt, A.; Stouvenel, L.; Lorès, P.; Ray, P.; Lousqui, J.; Pocate-Cheriet, K.; Santulli, P.; et al. Genetic diagnosis, sperm phenotype and ICSI outcome in case of severe asthenozoospermia with multiple morphological abnormalities of the flagellum. Hum. Reprod. 2021, 36, 2848–2860. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Li, L.; Zhou, L.M.; Le, F.; Cai, L.Y.; Yu, P.; Zhu, Y.R.; Liu, X.Z.; Wang, L.Y.; Li, L.J.; et al. Alterations in the frequency of trinucleotide repeat dynamic mutations in offspring conceived through assisted reproductive technology. Hum. Reprod. 2013, 28, 2570–2580. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, L.; Zeng, Y.; Kuang, Y.; Guan, Y.; Chen, B.; Xu, S.; Tang, B.; Wu, L.; Mao, X.; et al. Large-scale analysis of de novo mutations identifies risk genes for female infertility characterized by oocyte and early embryo defects. Genome Biol. 2023, 24, 68. [Google Scholar] [CrossRef]
- Xue, Y.; Cheng, X.; Xiong, Y.; Li, K. Gene mutations associated with fertilization failure after in vitro fertilization/intracytoplasmic sperm injection. Front. Endocrinol. 2022, 13, 1086883. [Google Scholar] [CrossRef]
- Hodžić, A.; Maver, A.; Plaseska-Karanfilska, D.; Ristanović, M.; Noveski, P.; Zorn, B.; Terzic, M.; Kunej, T.; Peterlin, B. De novo mutations in idiopathic male infertility—A pilot study. Andrology 2021, 9, 212–220. [Google Scholar] [CrossRef]
- Oud, M.S.; Smits, R.M.; Smith, H.E.; Mastrorosa, F.K.; Holt, G.S.; Houston, B.J.; de Vries, P.F.; Alobaidi, B.K.S.; Batty, L.E.; Ismail, H.; et al. A de novo paradigm for male infertility. Nat. Commun. 2022, 13, 154. [Google Scholar] [CrossRef]
- Smarr, M.M.; Sapra, K.J.; Gemmill, A.; Kahn, L.G.; Wise, L.A.; Lynch, C.D.; Factor-Litvak, P.; Mumford, S.L.; Skakkebaek, N.E.; Slama, R.; et al. Is human fecundity changing? A discussion of research and data gaps precluding us from having an answer. Hum. Reprod. 2017, 32, 499–504. [Google Scholar] [CrossRef]
- Nieschlag, E.; Lerchl, A. Sperm crisis: What crisis? Asian J. Androl. 2023, 15, 184–186. [Google Scholar] [CrossRef]
- Bonde, J.P.; te Velde, E. Declining sperm counts—The never-ending story. Nat. Rev. Urol. 2017, 14, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Ricci, E.; Chiaffarino, F.; Esposito, G.; Dalmartello, M.; La Vecchia, C.; Negri, E.; Parazzini, F. Trend of change of sperm count and concentration over the last two decades: A systematic review and meta-regression analysis. Andrology 2023, 11, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Mann, U.; Shiff, B.; Patel, P. Reasons for worldwide decline in male fertility. Curr. Opin. Urol. 2020, 30, 296–301. [Google Scholar] [CrossRef] [PubMed]
- te Velde, E.R.; Bonde, J.P. Misconceptions about falling sperm counts and fertility in Europe. Asian J. Androl. 2013, 15, 195–198. [Google Scholar] [CrossRef]
- Aitken, R.J.; Best, F.S.M.; Warner, P.; Templeton, A. A prospective study of the relationship between semen quality and fertility in cases of unexplained infertility. J. Androl. 1984, 5, 297–303. [Google Scholar] [CrossRef]
- Swan, S.H.; Colino, S. Count Down: How Our Modern World Is Threatening Sperm Counts, Altering Male and Female Reproductive Development, and Imperiling the Future of the Human Race; Simon and Schuster: New York, NY, USA, 2022. [Google Scholar]
- Priya, K.; Setty, M.; Babu, U.V.; Pai, K.S.R. Implications of environmental toxicants on ovarian follicles: How it can adversely affect the female fertility? Environ. Sci. Pollut. Res. Int. 2021, 28, 67925–67939. [Google Scholar] [CrossRef]
- Giudice, L.C. Environmental impact on reproductive health and risk mitigating strategies. Curr. Opin. Obstet. Gynecol. 2021, 33, 343–349. [Google Scholar] [CrossRef]
- Mukherjee, U.; Das, S.; Ghosh, S.; Maitra, S. Reproductive toxicity of bisphenol A, at environmentally relevant concentrations, on ovarian redox balance, maturational response, and intra-oocyte signalling events in Labeo bata. Sci. Total Environ. 2024, 906, 167415. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, S.; Jampy, A.; Moison, D.; Wieckowski, M.; Messiaen, S.; Martini, E.; Campalans, A.; Radicella, J.P.; Rouiller-Fabre, V.; Livera, G.; et al. Foetal exposure to the bisphenols BADGE and BPAF impairs meiosis through DNA oxidation in mouse ovaries. Environ. Pollut. 2023, 317, 120791. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, Y.-H.; Han, G.U.; Kim, S.G.; Bhang, D.H.; Kim, B.-G.; Moon, S.-H.; Shin, S.H.; Ryu, B.-Y. Diisobutyl phthalate (DiBP)-induced male germ cell toxicity and its alleviation approach. Food Chem. Toxicol. 2023, 184, 114387. [Google Scholar] [CrossRef]
- Sedha, S.; Kumar, S.; Shukla, S. Role of oxidative stress in male reproductive dysfunctions with reference to phthalate compounds. Urol. J. 2015, 12, 2304–2316. [Google Scholar]
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Vanage, G. Mutagenic effect of bisphenol A on adult rat male germ cells and their fertility. Reprod. Toxicol. 2013, 40, 60–68. [Google Scholar] [CrossRef]
- Jha, A.M.; Singh, A.C.; Bharti, M. Germ cell mutagenicity of phthalic acid in mice. Mutat. Res./Mol. Mech. Mutagen. 1998, 422, 207–212. [Google Scholar] [CrossRef]
- Shanmugam, D.A.S.; Dhatchanamurthy, S.; Leela, K.A.; Bhaskaran, R.S. Maternal exposure to di(2-ethylhexyl) phthalate (DEHP) causes multigenerational adverse effects on the uterus of F1 and F2 offspring rats. Reprod. Toxicol. 2023, 115, 17–28. [Google Scholar] [CrossRef]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; de Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Ziv-Gal, A.; Wang, W.; Zhou, C.; Flaws, J.A. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol. Appl. Pharmacol. 2015, 284, 354–362. [Google Scholar] [CrossRef]
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate exposure and long-term epigenomic consequences: A review. Front. Genet. 2020, 11, 405. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.A.; Yauk, C.L.; Marchetti, F. From sperm to offspring: Assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutat. Res./Rev. Mutat. Res. 2017, 773, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Omolaoye, T.S.; El Shahawy, O.; Skosana, B.T.; Boillat, T.; Loney, T.; du Plessis, S.S. The mutagenic effect of tobacco smoke on male fertility. Environ. Sci. Pollut. Res. Int. 2022, 29, 62055–62066. [Google Scholar] [CrossRef]
- Ji, B.-T.; Shu, X.-O.; Linet, M.S.; Zheng, W.; Wacholder, S.; Gao, Y.-T.; Ying, D.-M.; Jin, F. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J. Natl. Cancer Inst. 1997, 89, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Ward, M.H.; Han, S.; Ahn, H.S.; Kang, H.J.; Choi, H.S.; Shin, H.Y.; Koo, H.-H.; Seo, J.-J.; Choi, J.-E.; et al. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk. Res. 2009, 33, 250–258. [Google Scholar] [CrossRef]
- Axelsson, J.; Sabra, S.; Rylander, L.; Rignell-Hydbom, A.; Lindh, C.H.; Giwercman, A. Association between paternal smoking at the time of pregnancy and the semen quality in sons. PLoS ONE 2018, 13, e0207221. [Google Scholar] [CrossRef]
- Sobinoff, A.P.; Sutherland, J.M.; Beckett, E.L.; Stanger, S.J.; Johnson, R.; Jarnicki, A.G.; McCluskey, A.; St John, J.C.; Hansbro, P.M.; McLaughlin, E.A. Damaging legacy: Maternal cigarette smoking has long-term consequences for male offspring fertility. Hum. Reprod. 2014, 29, 2719–2735. [Google Scholar] [CrossRef]
- Wyrwoll, M.J.; van der Heijden, G.W.; Krausz, C.; Aston, K.I.; Kliesch, S.; McLachlan, R.; Ramos, L.; Conrad, D.F.; O’Bryan, M.K.; Veltman, J.A.; et al. Improved phenotypic classification of male infertility to promote discovery of genetic causes. Nat. Rev. Urol. 2024, 21, 91–101. [Google Scholar] [CrossRef]
- Van Der Kelen, A.; Okutman, Ö.; Javey, E.; Serdarogullari, M.; Janssens, C.; Ghosh, M.S.; Dequeker, B.J.H.; Perold, F.; Kastner, C.; Kieffer, E.; et al. A systematic review and evidence assessment of monogenic gene-disease relationships in human female infertility and differences in sex development. Hum. Reprod. Update 2023, 29, 218–232. [Google Scholar] [CrossRef]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Chambers, G.M.; Sullivan, E.A.; Ishihara, O.; Chapman, M.G.; Adamson, G.D. The economic impact of assisted reproductive technology: A review of selected developed countries. Fertil. Steril. 2009, 91, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Keiding, N.; Ali, M.M.; Eriksson, F.; Matsaseng, T.; Toskin, I.; Kiarie, J. The use of time to pregnancy for estimating and monitoring human fecundity from demographic and health surveys. Epidemiology 2021, 32, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.M.; Thoma, M.E.; Tchangalova, N.; Mburu, G.; Bornstein, M.J.; Johnson, C.L.; Kiarie, J. Infertility prevalence and the methods of estimation from 1990 to 2021: A systematic review and meta-analysis. Hum. Reprod. Open 2022, 2022, hoac051. [Google Scholar] [CrossRef]
- Bergsvik, J.; Fauske, A.; Hart, R.K. Can policies stall the fertility fall? A systematic review of the (quasi-) experimental literature. Popul. Dev. Rev. 2021, 47, 913–964. [Google Scholar] [CrossRef]
- Van Onselen, P.; Errington, W. Solutions and diversions: The debate on Australia’s ageing population. Aust. Q. 2004, 76, 6–10. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.J. The Global Decline in Human Fertility: The Post-Transition Trap Hypothesis. Life 2024, 14, 369. https://doi.org/10.3390/life14030369
Aitken RJ. The Global Decline in Human Fertility: The Post-Transition Trap Hypothesis. Life. 2024; 14(3):369. https://doi.org/10.3390/life14030369
Chicago/Turabian StyleAitken, Robert John. 2024. "The Global Decline in Human Fertility: The Post-Transition Trap Hypothesis" Life 14, no. 3: 369. https://doi.org/10.3390/life14030369
APA StyleAitken, R. J. (2024). The Global Decline in Human Fertility: The Post-Transition Trap Hypothesis. Life, 14(3), 369. https://doi.org/10.3390/life14030369