Microfluidics-Based Drying–Wetting Cycles to Investigate Phase Transitions of Small Molecules Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sunset Yellow Purification and Stock Solution Preparation
2.3. Microfluidic Chip Fabrication
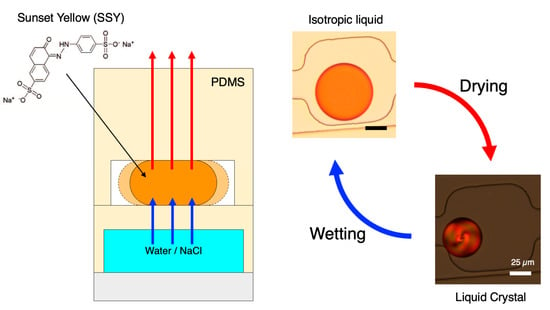
2.4. Microfluidic Chip Operation and Microscope Image Acquisition
2.5. Image Analysis
3. Results
3.1. Measurement of the SSY Phase Diagram
3.2. Implementation of Dry–Wet Cycles in the Microfluidic Device
3.3. Incubation of SSY Solutions in Different Phases by Controlled Drying
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Calculation of the Volume of a Droplet in the Microfluidic Device

References
- Follmann, H.; Brownson, C. Darwin’s Warm Little Pond Revisited: From Molecules to the Origin of Life. Naturwissenschaften 2009, 96, 1265–1292. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of First Cells at Terrestrial, Anoxic Geothermal Fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A.; Kim, H.J.; Carrigan, M.A. Asphalt, Water, and the Prebiotic Synthesis of Ribose, Ribonucleosides, and RNA. Acc. Chem. Res. 2012, 45, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. The Hot Spring Hypothesis for an Origin of Life. Astrobiology 2020, 20, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Baaske, P.; Weinert, F.M.; Duhr, S.; Lemke, K.H.; Russell, M.J.; Braun, D. Extreme Accumulation of Nucleotides in Simulated Hydrothermal Pore Systems. Proc. Natl. Acad. Sci. USA 2007, 104, 9346–9351. [Google Scholar] [CrossRef]
- Pearce, B.K.D.; Pudritz, R.E.; Semenov, D.A.; Henning, T.K. Origin of the RNA World: The Fate of Nucleobases in Warm Little Ponds. Proc. Natl. Acad. Sci. USA 2017, 114, 11327–11332. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Schneider, C.; Okamura, H.; Crisp, A.; Amatov, T.; Dejmek, M.; Carell, T. Wet-Dry Cycles Enable the Parallel Origin of Canonical and Non-Canonical Nucleosides by Continuous Synthesis. Nat. Commun. 2018, 9, 163. [Google Scholar] [CrossRef]
- Jia, T.Z.; Bellini, T.; Clark, N.; Fraccia, T.P. A Liquid Crystal World for the Origins of Life. Emerg. Top. Life Sci. 2022, 6, ETLS20220081. [Google Scholar] [CrossRef]
- Damer, B.; Deamer, D. Coupled Phases and Combinatorial Selection in Fluctuating Hydrothermal Pools: A Scenario to Guide Experimental Approaches to the Origin of Cellular Life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef]
- Pohorille, A.; Deamer, D. Self-Assembly and Function of Primitive Cell Membranes. Res. Microbiol. 2009, 160, 449–456. [Google Scholar] [CrossRef]
- Campbell, T.D.; Febrian, R.; McCarthy, J.T.; Kleinschmidt, H.E.; Forsythe, J.G.; Bracher, P.J. Prebiotic Condensation through Wet–Dry Cycling Regulated by Deliquescence. Nat. Commun. 2019, 10, 4508. [Google Scholar] [CrossRef] [PubMed]
- Olasagasti, F.; Rajamani, S. Lipid-Assisted Polymerization of Nucleotides. Life 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Vlassov, A.; Benner, S.; Coombs, A.; Olasagasti, F.; Deamer, D. Lipid-Assisted Synthesis of RNA-like Polymers from Mononucleotides. Orig. Life Evol. Biosph. 2008, 38, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Deguzman, V.; Vercoutere, W.; Shenasa, H.; Deamer, D. Generation of Oligonucleotides under Hydrothermal Conditions by Non-Enzymatic Polymerization. J. Mol. Evol. 2014, 78, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Pino, S.; Timperio, A.M.; Šponer, J.E.; Šponer, J.; Nováková, O.; Šedo, O.; Zdráhal, Z.; Di Mauro, E. Non-Enzymatic Oligomerization of 3′,5′ Cyclic AMP. PLoS ONE 2016, 11, e0165723. [Google Scholar] [CrossRef] [PubMed]
- Morasch, M.; Mast, C.B.; Langer, J.K.; Schilcher, P.; Braun, D. Dry Polymerization of 3′,5′-Cyclic GMP to Long Strands of RNA. ChemBioChem 2014, 15, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Dass, A.V.; Wunnava, S.; Langlais, J.; von der Esch, B.; Krusche, M.; Ufer, L.; Chrisam, N.; Dubini, R.C.A.; Gartner, F.; Angerpointner, S.; et al. RNA Oligomerisation without Added Catalyst from 2′,3′-Cyclic Nucleotides by Drying at Air-Water Interfaces. ChemSystemsChem 2022, 5, e202200026. [Google Scholar] [CrossRef]
- Wunnava, S.; Dirscherl, C.F.; Výravský, J.; Kovařík, A.; Matyášek, R.; Šponer, J.; Braun, D.; Šponer, J.E. Acid-Catalyzed RNA-Oligomerization from 3′,5′-CGMP. Chem.-A Eur. J. 2021, 27, 17581–17585. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Hill, A.R.; Liu, R.; Orgel, L.E. Synthesis of Long Prebiotic Oligomers on Mineral Surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef]
- Erastova, V.; Degiacomi, M.T.; Fraser, D.G.; Greenwell, H.C. Mineral Surface Chemistry Control for Origin of Prebiotic Peptides. Nat. Commun. 2017, 8, 2033. [Google Scholar] [CrossRef]
- Jerome, C.A.; Kim, H.J.; Mojzsis, S.J.; Benner, S.A.; Biondi, E. Catalytic Synthesis of Polyribonucleic Acid on Prebiotic Rock Glasses. Astrobiology 2022, 22, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.G.; Yu, S.S.; Mamajanov, I.; Grover, M.A.; Krishnamurthy, R.; Fernández, F.M.; Hud, N.V. Ester-Mediated Amide Bond Formation Driven by Wet-Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth. Angew. Chem. Int. Ed. 2015, 54, 9871–9875. [Google Scholar] [CrossRef] [PubMed]
- Martra, G.; Deiana, C.; Sakhno, Y.; Barberis, I.; Fabbiani, M.; Pazzi, M.; Vincenti, M. The Formation and Self-Assembly of Long Prebiotic Oligomers Produced by the Condensation of Unactivated Amino Acids on Oxide Surfaces. Angew. Chem. Int. Ed. 2014, 53, 4671–4674. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.D.; Solano, M.; Saydjari, A.; Bennett, C.J.; Hud, N.V.; Orlando, T.M. A Possible Path to Prebiotic Peptides Involving Silica and Hydroxy Acid-Mediated Amide Bond Formation. ChemBioChem 2018, 19, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Higgs, P.G. The Effect of Limited Diffusion and Wet–Dry Cycling on Reversible Polymerization Reactions: Implications for Prebiotic Synthesis of Nucleic Acids. Life 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing Life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Fujikawa, S.M.; Szostak, J.W. Experimental Models of Primitive Cellular Compartments: Encapsulation, Growth, and Division. Science 2003, 302, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A.; Szostak, J.W. A Kinetic Study of the Growth of Fatty Acid Vesicles. Biophys. J. 2004, 87, 988–998. [Google Scholar] [CrossRef]
- Mansy, S.S.; Szostak, J.W. Thermostability of Model Protocell Membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 13351–13355. [Google Scholar] [CrossRef]
- Deamer, D.; Singaram, S.; Rajamani, S.; Kompanichenko, V.; Guggenheim, S. Self-Assembly Processes in the Prebiotic Environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1809–1818. [Google Scholar] [CrossRef]
- Sarkar, S.; Dagar, S.; Verma, A.; Rajamani, S. Compositional Heterogeneity Confers Selective Advantage to Model Protocellular Membranes during the Origins of Cellular Life. Sci. Rep. 2020, 10, 4483. [Google Scholar] [CrossRef] [PubMed]
- Fares, H.M.; Marras, A.E.; Ting, J.M.; Tirrell, M.V.; Keating, C.D. Impact of Wet-Dry Cycling on the Phase Behavior and Compartmentalization Properties of Complex Coacervates. Nat. Commun. 2020, 11, 5423. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, F.P.; Choi, S.; Meyer, M.; Bevilacqua, P.; Keating, C. Prebiotically-Relevant Low Polyion Multivalency Can Improve Functionality of Membraneless Compartments. Nat. Commun. 2020, 11, 5949. [Google Scholar] [CrossRef] [PubMed]
- Fraccia, T.P.; Jia, T.Z. Liquid Crystal Coacervates Composed of Short Double-Stranded DNA and Cationic Peptides. ACS Nano 2020, 14, 15071–15082. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.Z.; Fraccia, T.P. Liquid Crystal Peptide/DNA Coacervates in the Context of Prebiotic Molecular Evolution. Crystals 2020, 10, 964. [Google Scholar] [CrossRef]
- Ghosh, B.; Bose, R.; Tang, T.Y.D. Can Coacervation Unify Disparate Hypotheses in the Origin of Cellular Life? Curr. Opin. Colloid Interface Sci. 2021, 52, 101415. [Google Scholar] [CrossRef]
- Fraccia, T.P.; Zanchetta, G. Liquid-Liquid Crystalline Phase Separation in Biomolecular Solutions. Curr. Opin. Colloid Interface Sci. 2021, 56, 101500. [Google Scholar] [CrossRef]
- Jia, T.Z.; Chandru, K.; Hongo, Y.; Afrin, R.; Usui, T.; Myojo, K. Membraneless Polyester Microdroplets as Primordial Compartments at the Origins of Life. Proc. Natl. Acad. Sci. USA 2019, 116, 15830–15835. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.Z.; Bapat, N.V.; Verma, A.; Mamajanov, I.; Cleaves, H.J.; Chandru, K. Incorporation of Basic α-Hydroxy Acid Residues into Primitive Polyester Microdroplets for RNA Segregation. Biomacromolecules 2021, 22, 1484–1493. [Google Scholar] [CrossRef]
- Nakata, M.; Zanchetta, G.; Chapman, B.D.; Jones, C.D.; Cross, J.O.; Pindak, R.; Bellini, T.; Clark, N.A. End-to-End Stacking and Liquid Crystal Condensation of 6 to 20 Base Pair DNA Duplexes. Science 2007, 318, 1276–1279. [Google Scholar] [CrossRef]
- Zanchetta, G.; Bellini, T.; Nakata, M.; Clark, N.A. Physical Polymerization and Liquid Crystallization of RNA Oligomers. J. Am. Chem. Soc. 2008, 130, 12864–12865. [Google Scholar] [CrossRef]
- Fraccia, T.P.; Smith, G.P.; Bethge, L.; Zanchetta, G.; Nava, G.; Klussmann, S.; Clark, N.A.; Bellini, T. Liquid Crystal Ordering and Isotropic Gelation in Solutions of Four-Base-Long DNA Oligomers. ACS Nano 2016, 10, 8508–8516. [Google Scholar] [CrossRef]
- Fraccia, T.P.; Smith, G.P.; Clark, N.A.; Bellini, T. Liquid Crystal Ordering of Four-Base-Long DNA Oligomers with Both G–C and A–T Pairing. Crystals 2018, 8, 5. [Google Scholar] [CrossRef]
- Todisco, M.; Smith, G.P.; Fraccia, T.P. Liquid Crystal Ordering of DNA Dickerson Dodecamer Duplexes with Different 5′-Phosphate Terminations. Mol. Cryst. Liq. Cryst. 2019, 683, 69–80. [Google Scholar] [CrossRef]
- Di Leo, S.; Todisco, M.; Bellini, T.; Fraccia, T.P. Phase Separations, Liquid Crystal Ordering and Molecular Partitioning in Mixtures of PEG and DNA Oligomers. Liq. Cryst. 2018, 45, 2306–2318. [Google Scholar] [CrossRef]
- Budin, I.; Szostak, J.W. Expanding Roles for Diverse Physical Phenomena during the Origin of Life. Annu. Rev. Biophys. 2010, 39, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Fraccia, T.P.; Zanchetta, G.; Rimoldi, V.; Clark, N.A.; Bellini, T. Evidence of Liquid Crystal–Assisted Abiotic Ligation of Nucleic Acids. Orig. Life Evol. Biosph. 2015, 45, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Bellini, T.; Zanchetta, G.; Fraccia, T.P.; Cerbino, R.; Tsai, E.; Smith, G.P.; Moran, M.J.; Walba, D.M.; Clark, N.A. Liquid Crystal Self-Assembly of Random-Sequence DNA Oligomers. Proc. Natl. Acad. Sci. USA 2012, 109, 1110–1115. [Google Scholar] [CrossRef]
- Di Leo, S.; Marni, S.; Plata, C.A.; Fraccia, T.P.; Smith, G.P.; Maritan, A.; Suweis, S.; Bellini, T. Pairing Statistics and Melting of Random DNA Oligomers: Finding Your Partner in Superdiverse Environments. PLoS Comput. Biol. 2022, 18, e1010051. [Google Scholar] [CrossRef]
- Fraccia, T.P.; Smith, G.P.; Zanchetta, G.; Paraboschi, E.; Yi, Y.; Walba, D.M.; Dieci, G.; Clark, N.A.; Bellini, T. Abiotic Ligation of DNA Oligomers Templated by Their Liquid Crystal Ordering. Nat. Commun. 2015, 6, 1611272. [Google Scholar] [CrossRef]
- Todisco, M.; Fraccia, T.P.; Smith, G.P.; Corno, A.; Bethge, L.; Klussmann, S.; Paraboschi, E.M.; Asselta, R.; Colombo, D.; Zanchetta, G.; et al. Nonenzymatic Polymerization into Long Linear RNA Templated by Liquid Crystal Self-Assembly. ACS Nano 2018, 12, 9750–9762. [Google Scholar] [CrossRef] [PubMed]
- Fraccia, T.P.; Martin, N. Non-Enzymatic Oligonucleotide Ligation in Coacervate Protocells Sustains Compartment-Content Coupling. Nat. Commun. 2023, 14, 2606. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Fraccia, T.P.; Todisco, M.; Zanchetta, G.; Zhu, C.; Hayden, E.; Bellini, T.; Clark, N.A. Backbone-Free Duplex-Stacked Monomer Nucleic Acids Exhibiting Watson–Crick Selectivity. Proc. Natl. Acad. Sci. USA 2018, 115, E7658–E7664. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.T.; Spada, G.P. Supramolecular Architectures Generated by Self-Assembly of Guanosine Derivatives. Chem. Soc. Rev. 2007, 36, 296–313. [Google Scholar] [CrossRef]
- Li, C.; Cafferty, B.J.; Karunakaran, S.C.; Schuster, G.B.; Hud, N.V. Formation of Supramolecular Assemblies and Liquid Crystals by Purine Nucleobases and Cyanuric Acid in Water: Implications for the Possible Origins of RNA. Phys. Chem. Chem. Phys. 2016, 18, 20091–20096. [Google Scholar] [CrossRef] [PubMed]
- Lydon, J. Chromonic Review. J. Mater. Chem. 2010, 20, 10071–10099. [Google Scholar] [CrossRef]
- Davidson, Z.S.; Huang, Y.; Gross, A.; Martinez, A.; Still, T.; Zhou, C.; Collings, P.J.; Kamien, R.D.; Yodh, A.G. Deposition and Drying Dynamics of Liquid Crystal Droplets. Nat. Commun. 2017, 8, 15642. [Google Scholar] [CrossRef]
- Horowitz, V.R.; Janowitz, L.A.; Modic, A.L.; Heiney, P.A.; Collings, P.J. Aggregation Behavior and Chromonic Liquid Crystal Properties of an Anionic Monoazo Dye. Phys. Rev. E-Stat. Nonlinear Soft Matter Phys. 2005, 72, 041710. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kang, S.W.; Tortora, L.; Nastishin, Y.; Finotello, D.; Kumar, S.; Lavrentovieh, O.D. Self-Assembly of Lyotropic Chromonic Liquid Crystal Sunset Yellow and Effects of Ionic Additives. J. Phys. Chem. B 2008, 112, 16307–16319. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Gruner, P.; Riechers, B.; Semin, B.; Lim, J.; Johnston, A.; Short, K.; Baret, J.-C. Controlling Molecular Transport in Minimal Emulsions. Nat. Commun. 2016, 7, 10392. [Google Scholar] [CrossRef] [PubMed]
- Selimović, E.; Gobeaux, F.; Fraden, S. Mapping and Manipulating Temperature-Concentration Phase Diagrams Using Microfluidics. Lab Chip 2010, 10, 1696–1699. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.U.; Cristobal, G.; Link, D.R.; Thorsen, T.; Jia, Y.; Piattelli, K.; Fraden, S. Control and Measurement of the Phase Behavior of Aqueous Solutions Using Microfluidics. J. Am. Chem. Soc. 2007, 129, 8825–8835. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Joanicot, M.; Ajdari, A. Microfluidic Exploration of the Phase Diagram of a Surfactant/Water Binary System. Langmuir 2007, 23, 2315–2317. [Google Scholar] [CrossRef] [PubMed]
- Kornreich, M.; Heymann, M.; Fraden, S.; Beck, R. Cross Polarization Compatible Dialysis Chip. Lab Chip 2014, 14, 3700–3704. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ong, I.L.H.; Sengupta, A. Time Dependent Lyotropic Chromonic Textures in Microfluidic Confinements. Crystals 2021, 11, 35. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Sengupta, A. Spatio-Temporal Programming of Lyotropic Phase Transition in Nanoporous Microfluidic Confinements. J. Colloid Interface Sci. 2023, 649, 302–312. [Google Scholar] [CrossRef]
- Tone, C.M.; Zizzari, A.; Spina, L.; Bianco, M.; De Santo, M.P.; Arima, V.; Barberi, R.C.; Ciuchi, F. Sunset Yellow Confined in Curved Geometry: A Microfluidic Approach. Langmuir 2023, 39, 6134–6141. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.M.; Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Consani, G.; Abell, C.; Vignolini, S. Hierarchical Self-Assembly of Cellulose Nanocrystals in a Confined Geometry. ACS Nano 2016, 10, 8443–8449. [Google Scholar] [CrossRef]
- He, K.; Zhou, Y.; Ramezani-Dakhel, H.; de Pablo, J.J.; Fernandez-Nieves, A.; Lopez-Leon, T. From Nematic Shells to Nematic Droplets: Energetics and Defect Transitions. Soft Matter 2022, 18, 1395–1403. [Google Scholar] [CrossRef]
- Dangla, R.; Lee, S.; Baroud, C.N. Trapping Microfluidic Drops in Wells of Surface Energy. Phys. Rev. Lett. 2011, 107, 124501. [Google Scholar] [CrossRef] [PubMed]
- Boukellal, H.; Selimović, E.; Jia, Y.; Cristobal, G.; Fraden, S. Simple, Robust Storage of Drops and Fluids in a Microfluidic Device. Lab Chip 2009, 9, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Randall, G.C.; Doyle, P.S. Permeation-Driven Flow in Poly(Dimethylsiloxane) Microfluidic Devices. Proc. Natl. Acad. Sci. USA 2005, 102, 10813–10818. [Google Scholar] [CrossRef] [PubMed]
- Toepke, M.W.; Beebe, D.J. PDMS Absorption of Small Molecules and Consequences in Microfluidic Applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef] [PubMed]






| Advantages | Disadvantages | |
|---|---|---|
| Chip 1 | Fast and controlled production of droplets, large droplets surface-to-volume ratio that increases the sensitivity of the measurement of the volume. | Not compatible with a reservoir layer because the high deformability of the thin PDMS membrane causes surface tension and traps destabilization. |
| Chip 2 | Fast and controlled production of droplets, compatibility of the trapping method with reservoir layer. | Lower droplets surface to volume ratio (i.e., less precision for phase diagram determination), too close traps and escaping of small (high concentration) droplets from traps (i.e., not suitable for parallel incubation of samples in different phases). |
| Chip 3 | Compatible with multiple reservoir channels, large droplets surface to volume ratio. | Slower and less controlled droplets production process, non-circular shape of the droplets, possible splitting/escaping of the droplets during the drying process (i.e., less suitable for phase diagram determination). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, A.; Mateo, T.; Quintero Botero, J.; Mohankumar, N.; Fraccia, T.P. Microfluidics-Based Drying–Wetting Cycles to Investigate Phase Transitions of Small Molecules Solutions. Life 2024, 14, 472. https://doi.org/10.3390/life14040472
Verma A, Mateo T, Quintero Botero J, Mohankumar N, Fraccia TP. Microfluidics-Based Drying–Wetting Cycles to Investigate Phase Transitions of Small Molecules Solutions. Life. 2024; 14(4):472. https://doi.org/10.3390/life14040472
Chicago/Turabian StyleVerma, Ajay, Tiphaine Mateo, Juan Quintero Botero, Nishanth Mohankumar, and Tommaso P. Fraccia. 2024. "Microfluidics-Based Drying–Wetting Cycles to Investigate Phase Transitions of Small Molecules Solutions" Life 14, no. 4: 472. https://doi.org/10.3390/life14040472
APA StyleVerma, A., Mateo, T., Quintero Botero, J., Mohankumar, N., & Fraccia, T. P. (2024). Microfluidics-Based Drying–Wetting Cycles to Investigate Phase Transitions of Small Molecules Solutions. Life, 14(4), 472. https://doi.org/10.3390/life14040472