Comparison of the Ultrasound Visibility of Tissue Markers in Metastatic Lymph Nodes after Neoadjuvant Chemotherapy in Patients with Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Tissue Marker Insertion for Axillary Lymph Nodes before NAC
2.3. Localization of Tissue Marker-Inserted Axillary Lymph Nodes after NAC
2.4. Pathological Correlation after the Surgery
2.5. Data Analysis
3. Results
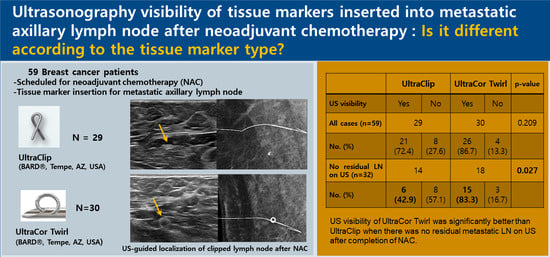
3.1. Comparison of the US Visibility between the Two Tissue Markers after NAC
3.2. Comparison of the Successful Excision Rates of the Clipped Lymph Nodes between the Two Tissue Markers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubens, R.D.; Sexton, S.; Tong, D.; Winter, P.J.; Knight, R.K.; Hayward, J.L. Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur. J. Cancer 1980, 16, 351–356. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Hortobagyi, G.N.; Rouzier, R.; Kuerer, H.; Sneige, N.; Buzdar, A.U.; Kau, S.W.; Fornage, B.; Sahin, A.; Broglio, K.; et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J. Clin. Oncol. 2005, 23, 9304–9311. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer (Version4. 2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 24 June 2022).
- Kuerer, H.M.; Sahin, A.A.; Hunt, K.K.; Newman, L.A.; Breslin, T.M.; Ames, F.C.; Ross, M.I.; Buzdar, A.U.; Hortobagyi, G.N.; Singletary, S.E. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann. Surg. 1999, 230, 72–78. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Ibrahim, N.K.; Francis, D.; Booser, D.J.; Thomas, E.S.; Theriault, R.L.; Pusztai, L.; Green, M.C.; Arun, B.K.; Giordano, S.H.; et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J. Clin. Oncol. 2005, 23, 3676–3685. [Google Scholar] [CrossRef] [PubMed]
- Dominici, L.S.; Negron Gonzalez, V.M.; Buzdar, A.U.; Lucci, A.; Mittendorf, E.A.; Le-Petross, H.T.; Babiera, G.V.; Meric-Bernstam, F.; Hunt, K.K.; Kuerer, H.M. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer 2010, 116, 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy in Patients With Node-Positive Breast Cancer: The ACOSOG Z1071 (Alliance) Clinical Trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilewskie, M.; Morrow, M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol. 2017, 3, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef]
- Shin, K.; Caudle, A.S.; Kuerer, H.M.; Santiago, L.; Candelaria, R.P.; Dogan, B.; Leung, J.; Krishnamurthy, S.; Yang, W. Radiologic Mapping for Targeted Axillary Dissection: Needle Biopsy to Excision. AJR Am. J. Roentgenol. 2016, 207, 1372–1379. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hieken, T.J.; Glazebrook, K.N.; Boughey, J.C. Localizing the Clipped Node in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy: Early Learning Experience and Challenges. Ann. Surg. Oncol. 2017, 24, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.H.; Teo, S.Y.; Gudi, M.; Ng, R.P.; Pang, J.; Tan, Y.S.; Lee, Y.S.; Allen, J.C., Jr.; Leong, L.C.H. Initial results of a novel technique of clipped node localization in breast cancer patients postneoadjuvant chemotherapy: Skin Mark clipped Axillary nodes Removal Technique (SMART trial). Cancer Med. 2020, 9, 1978–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portnow, L.H.; Kwak, E.; Senapati, G.M.; Kwait, D.C.; Denison, C.M.; Giess, C.S. Ultrasound visibility of select breast biopsy markers for targeted axillary node localization following neoadjuvant treatment: Simulation using animal tissue models. Breast Cancer Res. Treat. 2020, 184, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Kim, E.K.; Moon, H.J.; Yoon, J.H.; Park, V.Y.; Kim, M.J. Comparison of breast tissue markers for tumor localization in breast cancer patients undergoing neoadjuvant chemotherapy. Ultrasonography 2019, 38, 336–344. [Google Scholar] [CrossRef] [PubMed]





| Total (n = 59) | UltraClip (n = 29) | UltraCor Twirl (n = 30) | p-Value | |
|---|---|---|---|---|
| Age a, years, mean (SD) | 49.2 (10.3) | 49.3 (10.1) | 49.0 (10.6) | 0.899 b |
| Tumor stage, n (%) | 0.087 c | |||
| IB | 16 (27.1) | 3 (10.3) | 13 (43.3) | |
| IIA | 8 (13.6) | 4 (13.8) | 4 (13.3) | |
| IIB | 11 (18.6) | 7 (24.1) | 4 (13.3) | |
| IIIA | 11 (18.6) | 6 (20.7) | 5 (16.7) | |
| IIIB | 5 (8.5) | 4 (13.8) | 1 (3.3) | |
| IIIC | 8 (13.6) | 5 (17.2) | 3 (10.0) | |
| Tumor subtype, n (%) | 0.328 d | |||
| ER | 23 (39.0) | 9 (31.0) | 13 (46.7) | |
| Her-2 | 21 (35.6) | 13 (44.8) | 8 (26.7) | |
| TNBC | 15 (25.4) | 7 (24.1) | 8 (26.7) | |
| Breast surgery, n (%) | 0.235 d | |||
| BCS | 32 (54.2) | 18 (62.1) | 14 (46.7) | |
| TM | 27 (45.8) | 11 (37.9) | 16 (53.3) | |
| Axillary surgery, n (%) | 0.228 d | |||
| TAD | 34 (57.6) | 19 (65.5) | 15 (50.0) | |
| TAD with ALND | 25 (42.4) | 10 (34.5) | 15 (50.0) | |
| Localization, n (%) | 0.824 d | |||
| Yes | 44 (74.6) | 22 (75.9) | 22 (73.3) | |
| No | 15 (25.4) | 7 (24.1) | 8 (26.7) |
| UltraClip | UltraCor Twirl | p-Value | |||
|---|---|---|---|---|---|
| US Visibility | Yes | No | Yes | No | |
| All cases (n = 59) | 29 | 30 | 0.209 a | ||
| No. (%) | 21 (72.4) | 8 (27.6) | 26 (86.7) | 4 (13.3) | |
| No residual LN on US (n = 32) | 14 | 18 | 0.027 a | ||
| No. (%) | 6 (42.9) | 8 (57.1) | 15 (83.3) | 3 (16.7) | |
| UltraClip | UltraCor Twirl | p-Value | |||
|---|---|---|---|---|---|
| Successful Excision | Yes | No | Yes | No | |
| All cases (n = 58) | 29 | 29 | 1.000 a | ||
| No. (%) | 26 (89.7) | 3 (10.3) | 25 (86.2) | 4 (13.8) | |
| With localization (n = 43) | 22 | 21 | 0.233 a | ||
| No. (%) | 22 (100.0) | 0 (0) | 19 (90.5) | 3 (14.3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.E.; Ko, E.Y.; Han, B.-K.; Ko, E.S.; Choi, J.S.; Kim, H.; Lee, J.E.; Lee, H. Comparison of the Ultrasound Visibility of Tissue Markers in Metastatic Lymph Nodes after Neoadjuvant Chemotherapy in Patients with Breast Cancer. Diagnostics 2022, 12, 2424. https://doi.org/10.3390/diagnostics12102424
Kim KE, Ko EY, Han B-K, Ko ES, Choi JS, Kim H, Lee JE, Lee H. Comparison of the Ultrasound Visibility of Tissue Markers in Metastatic Lymph Nodes after Neoadjuvant Chemotherapy in Patients with Breast Cancer. Diagnostics. 2022; 12(10):2424. https://doi.org/10.3390/diagnostics12102424
Chicago/Turabian StyleKim, Ka Eun, Eun Young Ko, Boo-Kyung Han, Eun Sook Ko, Ji Soo Choi, Haejung Kim, Jeong Eon Lee, and Hyunwoo Lee. 2022. "Comparison of the Ultrasound Visibility of Tissue Markers in Metastatic Lymph Nodes after Neoadjuvant Chemotherapy in Patients with Breast Cancer" Diagnostics 12, no. 10: 2424. https://doi.org/10.3390/diagnostics12102424
APA StyleKim, K. E., Ko, E. Y., Han, B. -K., Ko, E. S., Choi, J. S., Kim, H., Lee, J. E., & Lee, H. (2022). Comparison of the Ultrasound Visibility of Tissue Markers in Metastatic Lymph Nodes after Neoadjuvant Chemotherapy in Patients with Breast Cancer. Diagnostics, 12(10), 2424. https://doi.org/10.3390/diagnostics12102424