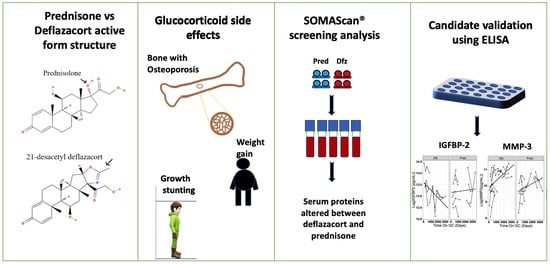
Comparison of Serum Pharmacodynamic Biomarkers in Prednisone-Versus Deflazacort-Treated Duchenne Muscular Dystrophy Boys
Abstract
:1. Introduction
2. Materials and Methods
2.1. SOMAscan® Dataset
2.2. Validation of Key Pharmacodynamic Biomarkers Using ELISA
2.3. Data Analysis and Statistical Methods
3. Results
3.1. Longitudinal Trajectory of Serum PD Biomarkers in Prednisone vs. Deflazacort Treated DMD boys
3.2. Effect of Dfz and Pred on Height and Weight of DMD Boys
3.3. Data Validation Using ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Wallace, G.Q.; McNally, E.M. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu. Rev. Physiol. 2009, 71, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.J. Ataluren: First global approval. Drugs 2014, 74, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Rodino-Klapac, L.R.; Sahenk, Z.; Roush, K.; Bird, L.; Lowes, L.P.; Alfano, L.; Gomez, A.M.; Lewis, S.; Kota, J.; et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 2013, 74, 637–647. [Google Scholar] [CrossRef]
- Syed, Y.Y. Eteplirsen: First global approval. Drugs 2016, 76, 1699–1704. [Google Scholar] [CrossRef]
- Heo, Y.A. Golodirsen: First approval. Drugs 2020, 80, 329–333. [Google Scholar] [CrossRef]
- Frank, D.E.; Schnell, F.J.; Akana, C.; El-Husayni, S.H.; Desjardins, C.A.; Morgan, J.; Charleston, J.S.; Sardone, V.; Domingos, J.; Dickson, G.; et al. Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology 2020, 94, e2270–e2282. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, E.P.; Bronson, A.; Levin, A.A.; Takeda, S.; Yokota, T.; Baudy, A.R.; Connor, E.M. Restoring dystrophin expression in Duchenne muscular dystrophy muscle: Progress in exon skipping and stop codon read through. Am. J. Pathol. 2011, 179, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Griggs, R.C.; Miller, J.P.; Greenberg, C.R.; Fehlings, D.L.; Pestronk, A.; Mendell, J.R.; Moxley, R.T.; King, W.; Kissel, J.T.; Cwik, V.; et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology 2016, 87, 2123–2131. [Google Scholar] [CrossRef] [Green Version]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Duong, T.; Joyce, N.C.; Hu, F.; Clemens, P.R.; Hoffman, E.P.; Cnaan, A.; Gordish-Dressman, H.; et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: A prospective cohort study. Lancet 2018, 391, 451–461. [Google Scholar] [CrossRef]
- Bello, L.; Gordish-Dressman, H.; Morgenroth, L.P.; Henricson, E.K.; Duong, T.; Hoffman, E.P.; Cnaan, A.; McDonald, C.M. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology 2015, 85, 1048–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, M.M.; West, N.A.; Ouyang, L.; Yang, M.; Weitzenkamp, D.; James, K.; Ciafaloni, E.; Pandya, S.; DiGuiseppi, C.; Cunniff, C.; et al. Corticosteroid treatment and growth patterns in ambulatory males with Duchenne muscular dystrophy. J. Pediatr. 2016, 173, 207–213.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifati, M.D.; Ruzza, G.; Bonometto, P.; Berardinelli, A.; Gorni, K.; Orcesi, S.; Lanzi, G.; Angelini, C. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve 2000, 23, 1344–1347. [Google Scholar] [CrossRef]
- Cohen, L.; Morgan, J.; Bozyk, M. Variable effects of corticosteroid treatment of serum enzyme activities in Duchenne’s muscular dystrophy. Res. Commun. Chem. Pathol. Pharmacol. 1977, 17, 529–538. [Google Scholar]
- FDA News Release (2017) FDA Approves Drug to Treat Duchenne Muscular Dystrophy. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-drug-treat-duchenne-muscular-dystrophy (accessed on 1 September 2020).
- Hathout, Y.; Liang, C.; Ogundele, M.; Xu, G.; Tawalbeh, S.M.; Dang, U.J.; Hoffman, E.P.; Gordish-Dressman, H.; Conklin, L.S.; van den Anker, J.N.; et al. Disease-specific and glucocorticoid-responsive serum biomarkers for Duchenne muscular dystrophy. Sci. Rep. 2019, 9, 12167. [Google Scholar] [CrossRef]
- Dang, U.J.; Ziemba, M.; Clemens, P.R.; Hathout, Y.; Conklin, L.S.; Hoffman, E.P. Serum biomarkers associated with baseline clinical severity in young steroid-naïve Duchenne muscular dystrophy boys. Hum. Mol. Genet. 2020, 29, 2481–2495. [Google Scholar] [CrossRef]
- Spitali, P.; Hettne, K.; Tsonaka, R.; Charrout, M.; van den Bergen, J.; Koeks, Z.; Kan, H.E.; Hooijmans, M.T.; Roos, A.; Straub, V.; et al. Tracking disease progression non-invasively in Duchenne and Becker muscular dystrophies. J. Cachexia. Sarcopenia Muscle 2018, 9, 715–726. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Tucker, P.; Mushtaq, T.; Wallace, A.M.; Williams, D.M.; Hughes, I.A. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin. Endocrinol. 2002, 57, 185–191. [Google Scholar] [CrossRef]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [Green Version]
- McDonald, C.M.; Sajeev, G.; Yao, Z.; McDonnell, E.; Elfring, G.; Souza, M.; Peltz, S.W.; Darras, B.T.; Shieh, P.B.; Cox, D.A.; et al. Deflazacort vs prednisone treatment for Duchenne muscular dystrophy: A meta-analysis of disease progression rates in recent multicenter clinical trials. Muscle Nerve. 2020, 61, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganapati, A.; Ravindran, R.; David, T.; Yadav, B.; Jeyaseelan, V.; Jeyaseelan, L.; Danda, D. Head to head comparison of adverse effects and efficacy between high dose deflazacort and high dose prednisolone in systemic lupus erythematosus: A prospective cohort study. Lupus 2018, 27, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Hathout, Y.; Conklin, L.S.; Seol, H.; Gordish-Dressman, H.; Brown, K.J.; Morgenroth, L.P.; Nagaraju, K.; Heier, C.R.; Damsker, J.M.; van den Anker, J.N.; et al. Serum pharmacodynamic biomarkers for chronic corticosteroid treatment of children. Sci. Rep. 2016, 6, 31727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conklin, L.S.; Merkel, P.A.; Pachman, L.M.; Parikh, H.; Tawalbeh, S.; Damsker, J.M.; Cuthbertson, D.D.; Morgan, G.A.; Monach, P.A.; Hathout, Y.; et al. Serum biomarkers of glucocorticoid response and safety in anti-neutrophil cytoplasmic antibody-associated vasculitis and juvenile dermatomyositis. Steroids 2018, 140, 159–166. [Google Scholar] [CrossRef]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Han, J.J.; Escolar, D.M.; Florence, J.M.; Duong, T.; Arrieta, A.; Clemens, P.R.; Hoffman, E.P.; et al. The cooperative international neuromuscular research group duchenne natural history study-a longitudinal investigation in the era of glucocorticoid therapy: Design of protocol and the methods used. Muscle Nerve 2013, 48, 32–54. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Patel, S.R.; Cheng, X.; Cho, E.A.; Levitan, I.; Ullenbruch, M.; Phan, S.H.; Park, J.M.; Dressler, G.R. Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat. Med. 2005, 11, 387–393. [Google Scholar] [CrossRef]
- Zatorski, H.; Marynowski, M.; Fichna, J. Is insulin-like growth factor 1 (IGF-1) system an attractive target inflammatory bowel diseases? Benefits and limitation of potential therapy. Pharmacol. Rep. 2016, 68, 809–815. [Google Scholar] [CrossRef]
- Serrano, A.L.; Muñoz-Cánoves, P. Fibrosis development in early-onset muscular dystrophies: Mechanisms and translational implications. Semin. Cell Dev. Biol. 2017, 64, 181–190. [Google Scholar] [CrossRef]
- Selvin, E.; Halushka, M.K.; Rawlings, A.M.; Hoogeveen, R.C.; Ballantyne, C.M.; Coresh, J.; Astor, B.C. SRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 2013, 62, 2116–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanida, S.; Mizoshita, T.; Ozeki, K.; Katano, T.; Kataoka, H.; Kamiya, T.; Joh, T. Advances in refractory ulcerative colitis treatment: A new therapeutic target, Annexin A2. World J. Gastroenterol. 2015, 21, 8776–8786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Jiang, Y.; Chung, J.Y.; Li, Y.; Yu, Z.; Kim, J.W.; Lok, J.M.; Whalen, M.J.; Wang, X. Annexin A2 deficiency exacerbates neuroinflammation and long-term neurological deficits after traumatic brain injury in mice. Int. J. Mol. Sci. 2019, 20, 6125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defour, A.; Medikayala, S.; Van der Meulen, J.H.; Hogarth, M.W.; Holdreith, N.; Malatras, A.; Duddy, W.; Boehler, J.; Nagaraju, K.; Jaiswal, J.K. Annexin A2 links poor myofiber repair with inflammation and adipogenic replacement of the injured muscle. Hum. Mol. Genet. 2017, 26, 1979–1991. [Google Scholar] [CrossRef] [Green Version]
- Grønbæk, H.; Kreutzfeldt, M.; Kazankov, K.; Jessen, N.; Sandahl, T.; Hamilton-Dutoit, S.; Vilstrup, H.; Møller, H.J. Single-centre experience of the macrophage activation marker soluble (s)CD163–associations with disease activity and treatment response in patients with autoimmune hepatitis. Aliment. Pharmacol. Ther. 2016, 44, 1062–1070. [Google Scholar]
- Espinasse, M.-A.; Pépin, A.; Virault-Rocroy, P.; Szely, N.; Chollet-Martin, S.; Pallardy, M.; Biola-Vidamment, A. Glucocorticoid-induced leucine zipper is expressed in human neutrophils and promotes apoptosis through Mcl-1 down-regulation. J. Innate Immun. 2016, 8, 81–96. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, P.; Xu, Z.; Chen, H.; Xie, X. GPNMB enhances bone regeneration by promoting angiogenesis and osteogenesis: Potential role for tissue engineering bone. J. Cell. Biochem. 2013, 114, 2729–2737. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, B.; Yao, Y. Prospects of osteoactivin in tissue regeneration. Expert Opin. Ther. Targets 2016, 20, 1357–1364. [Google Scholar] [CrossRef]
- Belongie, K.J.; Ferrannini, E.; Johnson, K.; Andrade-Gordon, P.; Hansen, M.K.; Petrie, J.R. Identification of novel biomarkers to monitor β-cell function and enable early detection of type 2 diabetes risk. PLoS ONE 2017, 12, e0182932. [Google Scholar] [CrossRef]
- DeMambro, V.E.; Clemmons, D.R.; Horton, L.G.; Bouxsein, M.L.; Wood, T.L.; Beamer, W.G.; Canalis, E.; Rosen, C.J. Gender-specific changes in bone turnover and skeletal architecture in Igfbp-2-null mice. Endocrinology 2008, 149, 2051–2061. [Google Scholar] [CrossRef]
- Hahn, B.H.; MacDermott, R.P.; Jacobs, S.B.; Pletscher, L.S.; Beale, M.G. Immunosuppressive effects of low doses of glucocorticoids: Effects on autologous and allogeneic mixed leukocyte reactions. J. Immunol. 1980, 124, 2812–2817. [Google Scholar] [PubMed]
- Lippuner, K.; Casez, J.-P.; Horber, F.F.; Jaeger, P. Effects of deflazacort versus prednisone on bone mass, body composition, and lipid profile: A randomized, double blind study in kidney transplant patients. J. Clin. Endocrinol. Metab. 1998, 83, 3795–3802. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Lombardi, A.; Ferraris, G.M.; Imbimbo, B.; Cavallo Perin, P. Acute effect of prednisone and deflazacort on glucose tolerance in prediabetic subjects. Eur. J. Clin. Pharmacol. 1982, 22, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Kemaladewi, D.U.; Pasteuning, S.; Van Der Meulen, J.W.; Van Heiningen, S.H.; Van Ommen, G.J.; Ten Dijke, P.; Aartsma-Rus, A.; ’T Hoen, P.A.C.; Hoogaars, W.M. Targeting TGF-β signaling by antisense oligonucleotide-mediated knockdown of TGF-β type I receptor. Mol. Ther.-Nucleic Acids 2014, 3, e156. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Wang, C.; Bushby, K.; Guglieri, M.; Horrocks, I.; Straub, V.; Ahmed, S.F.; Wong, S.C. Fractures and linear growth in a nationwide cohort of boys with Duchenne muscular dystrophy with and without glucocorticoid treatment: Results from the UK Northstar database. JAMA Neurol. 2019, 76, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Marden, J.R.; Freimark, J.; Yao, Z.; Signorovitch, J.; Tian, C.; Wong, B.L. Real-world outcomes of long-term prednisone and deflazacort use in patients with Duchenne muscular dystrophy: Experience at a single, large care center. J. Comp. Eff. Res. 2020, 9, 177–189. [Google Scholar] [CrossRef]
- Conklin, L.S.; Damsker, J.M.; Hoffman, E.P.; Jusko, W.J.; Mavroudis, P.D.; Schwartz, B.D.; Mengle-Gaw, L.J.; Smith, E.C.; Mah, J.K.; Guglieri, M.; et al. Phase IIa trial in Duchenne muscular dystrophy shows vamorolone is a first-in-class dissociative steroidal anti-inflammatory drug. Pharmacol. Res. 2018, 136, 140–150. [Google Scholar] [CrossRef]





| Treatment | Number of Patients | Average Number of Visits (Min, Max) | Age Range at Sample Collection (Years) | Average Time between Biosample Collection (Days) (Mix, Max) | Regimen |
|---|---|---|---|---|---|
| Deflazacort | 8 | 2.5 (2, 4) | 4.7–9.4 | 465 (56, 1268) | Daily on Dfz |
| Prednisone | 7 | 2.14 (2, 3) | 4.3–8.3 | 582 (157, 1392) | Daily on Pred |
| Treatment | Number of Patients | Average Number of Visits (Min, Max) | Mean Age at Sample Collection in Years (Min-Max) | Average Time between Biosample Collection (Days) (Mix, Max) |
|---|---|---|---|---|
| Deflazacort | 17 | 2.7 (2, 5) | 9 (4.7–15.3) | 1392 (370, 3058) |
| Prednisone | 12 | 2.5 (2, 3) | 8.5 (4.2–15.8) | 1685 (594, 3391) |
| Abbreviated Gene Name 1 (Uniprot ID) | Fold Change between Untreated DMD vs. Healthy Controls with p-Value from [17] | Fold-Change between DMD Subjects Treated with Pred and Dfz | p-Value: Difference in Mean Levels 2 | p-Value: Difference in Trajectories 2 | Protein Function—Biological Process | Potential Significance |
|---|---|---|---|---|---|---|
| LILRB1 (Q8NHL6) | 0.96 (0.861) | −1.5 (↓) in Pred vs. Dfz | 0.005 | 0.426 | Immune response | Side effect (immune suppression) |
| TNFRSF21 (O75509) | 1.14 (0.454) | −1.33 (↓) in Pred vs. Dfz | 0.005 | 0.934 | Apoptotic process, adaptive immune response | Side effect (immune suppression) |
| CHRDL1 (Q9BU40) | 1.30 (0.039) | −1.2 (↓) in Pred vs. Dfz | 0.007 | 0.455 | Bone Morphogenetic Proteins (BMP) signaling pathway | Efficacy via action on TGF-β signaling [30] |
| IGF-I (P05019) | 0.85 (0.161) | 1.14 (↑) in Pred vs. Dfz | 0.007 | 0.096 | Promotes growth | Potential efficacy associated with anti-inflammatory propriety [31] |
| MMP-3 (P08254) | 0.77 (0.488) | 2 (↑) in Dfz vs. Pred | 0.008 | 0.216 | Extracellular matrix degradation | Efficacy/Side effect [17,25,32] |
| sRAGE/AGER (Q15109) | 0.60 (0.005) | −1.82 (↓) in Pred vs. Dfz | 0.010 | 0.640 | Inflammatory (causes complications in diabetes) | Side effect may be associated with diabetes risk [33] |
| ANXA2 (P07355) | 1.24 (0.246) | −1.22 (↓) in Pred vs. Dfz | 0.011 | 0.054 | Angiogenesis, biomineral tissue development, inflammation | Potential efficacy marker associated with inflammation [34,35,36] |
| CD166 (Q13740) | 0.81 (0.088) | −1.25 (↓) in Pred vs. Dfz | 0.014 | 0.616 | Cell adhesion, adaptive immune response | Side effect (immune suppression) |
| HJV (Q6ZVN8) | 0.83 (0.073) | 1.19 (↑) in Dfz vs. Pred | 0.017 | 0.385 | BMP signaling pathway, iron ion homeostasis | Unknown |
| sCD163 (Q86VB7) | 0.86 (0.466) | −1.30 (↓) in Pred vs. Dfz | 0.025 | 0.340 | Inflammation | Efficacy [37] |
| Mcl-1 (Q07820) | 2.31 (<0.001) | −1.33 (↓) in Pred vs. Dfz | 0.029 | 0.146 | Apoptosis, DifferentiationInflammation | Efficacy [38] |
| PDE3A (Q14432) | 1.03 (0.805) | 1.22 (↑) in Pred vs. Dfz | 0.033 | 0.956 | Cell to cell signaling | Unknown |
| GPNMB (Q14956) | 0.79 (0.111) | −1.32 (↓) in Pred vs. Dfz | 0.033 | 0.944 | Cell adhesion, bone mineralization. | Side effect related to bone [39,40] |
| FCN1 (O00602) | 1.02 (0.905) | 1.18 (↑) in Pred vs. Dfz | 0.038 | 0.030 | Innate immune response | Unknown |
| MAPK14 (Q16539) | 2.10 (<0.001) | −1.18 (↓) in Pred vs. Dfz | 0.041 | 0.982 | Potential muscle injury biomarker | Efficacy, muscle injury protein that normalized after GC treatment [17] |
| NCAM-L1 (P32004) | 0.76 (0.072) | −1.28 (↓) in Pred vs. Dfz | 0.042 | 0.691 | Cell adhesion and differentiation | Side effect, risk of developing diabetes [41] |
| IGFBP-2 (P18065) | 2.55 (<0.001) | −1.16 (↓) in Dfz vs. Pred | 0.329 | 0.040 | Growth regulation | Side effect, growth stunting [42] |
| Protein Name (Uniprot ID) | SOMAscan® Data | ELISA Data | Function | ||||
|---|---|---|---|---|---|---|---|
| p-Value 1: Difference in Mean Levels | p-Value 1: Difference in Trajectories | Number of Patients/Samples | p-Value 1: Difference in Mean Levels | p-Value 1: Difference in Trajectories | Number of Patients/Samples | ||
| MMP-3 (P08254) | 0.008 | 0.216 | 8 Dfz, 7 Pred/35 samples | 0.022 | 0.378 | 17 Dfz, 12 Pred/76 Samples | Extracellular matrix degradation |
| IGFBP-2 (P18065) | 0.328 | 0.04 | 8 Dfz, 7 Pred/35 samples | 0.744 | 0.0507 | 10 Dfz, 10 Pred/49 Samples | Regulates growth |
| IGF-I (P05019) | 0.007 | 0.096 | 8 Dfz, 7 Pred/35 samples | 0.246 | 0.137 | 17 Dfz, 12 Pred/75 Samples | Promotes growth |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawalbeh, S.; Samsel, A.; Gordish-Dressman, H.; Hathout, Y.; CINRG-DNHS Investigators; Dang, U.J. Comparison of Serum Pharmacodynamic Biomarkers in Prednisone-Versus Deflazacort-Treated Duchenne Muscular Dystrophy Boys. J. Pers. Med. 2020, 10, 164. https://doi.org/10.3390/jpm10040164
Tawalbeh S, Samsel A, Gordish-Dressman H, Hathout Y, CINRG-DNHS Investigators, Dang UJ. Comparison of Serum Pharmacodynamic Biomarkers in Prednisone-Versus Deflazacort-Treated Duchenne Muscular Dystrophy Boys. Journal of Personalized Medicine. 2020; 10(4):164. https://doi.org/10.3390/jpm10040164
Chicago/Turabian StyleTawalbeh, Shefa, Alison Samsel, Heather Gordish-Dressman, Yetrib Hathout, CINRG-DNHS Investigators, and Utkarsh J. Dang. 2020. "Comparison of Serum Pharmacodynamic Biomarkers in Prednisone-Versus Deflazacort-Treated Duchenne Muscular Dystrophy Boys" Journal of Personalized Medicine 10, no. 4: 164. https://doi.org/10.3390/jpm10040164
APA StyleTawalbeh, S., Samsel, A., Gordish-Dressman, H., Hathout, Y., CINRG-DNHS Investigators, & Dang, U. J. (2020). Comparison of Serum Pharmacodynamic Biomarkers in Prednisone-Versus Deflazacort-Treated Duchenne Muscular Dystrophy Boys. Journal of Personalized Medicine, 10(4), 164. https://doi.org/10.3390/jpm10040164