A Network-Based Mixed Methods Approach to Analyze Current Perspectives on Personalized Oncological Medicine in Austria
Abstract
:1. Introduction
2. Materials and Methods
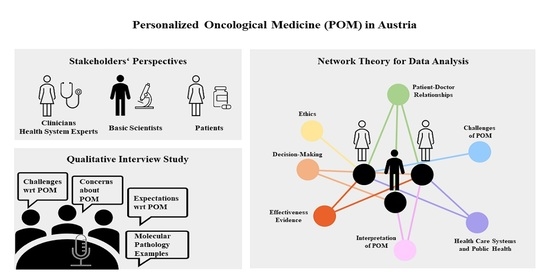
2.1. Qualitative Data Collection
2.2. Data Analysis and Visualization
3. Results and Discussion
3.1. Description of the Qualitative Interview Data
3.2. Network Analysis of the Qualitative Interview Data
3.2.1. Ethical Concerns
3.2.2. Concerns about Progress in Cancer Research
3.2.3. Concerns about Data Collection
3.2.4. Concerns about Costs and Health Economics
3.2.5. Concerns about RCTs
3.2.6. Concerns about Knowledge Gaps, Translational Issues and Communication Issues
3.2.7. Concerns about Patient-Doctor Relationships
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Official Journal of the European Union, C 421, 16 November 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=OJ%3AC%3A2016%3A421%3ATOC (accessed on 15 November 2020).
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilsky, R.L. Personalized medicine in oncology: The future is now. Nat. Rev. Drug. Discov. 2010, 9, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Sedda, G.; Gasparri, R.; Spaggiari, L. Challenges and innovations in personalized medicine care. Future Oncol. 2019, 15, 3305–3308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhavan, S.; Subramaniam, S.; Brown, T.D.; Chen, J.L. Art and challenges of precision medicine: Interpreting and integrating genomic data into clinical practice. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M. Personalized oncology: Recent advances and future challenges. Metabolism 2013, 62, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Dietel, M. Molecular pathology: A requirement for precision medicine in cancer. Oncol. Res. Treat. 2016, 39, 804–810. [Google Scholar] [CrossRef]
- Plönes, T.; Engel-Riedel, W.; Stoelben, E.; Limmroth, C.; Schildgen, O.; Schildgen, V. Molecular pathology and personalized medicine: The dawn of a new era in companion diagnostics—practical considerations about companion diagnostics for non-small-cell-lung-cancer. J. Pers. Med. 2016, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.S.; Beyer, T.; Babayan, A.; Bergmann, M.; Brehme, M.; Buyx, A.; Czernin, J.; Egger, G.; Elenitoba-Johnson, K.S.J.; Gückel, B.; et al. Advancing biomarker development through convergent engagement: Summary report of the 2nd international Danube symposium on biomarker development, molecular imaging and applied diagnostics; 14–16 March 2018; Vienna, Austria. Mol. Imaging Biol. 2020, 22, 47–65. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.; Lipworth, W.; Kerridge, I. Ethics, evidence and economics in the pursuit of “personalized medicine”. J. Pers. Med. 2014, 4, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, E.; Annemans, L.; Garrison, L.; Helfand, M.; Holtorf, A.-P.; Hornberger, J.; Hughes, D.; Li, T.; Malone, D.; Payne, K. Challenges in the development and reimbursement of personalized medicine—payer and manufacturer perspectives and implications for health economics and outcomes research: A report of the ISPOR Personalized Medicine Special Interest Group. Value Health 2012, 15, 1162–1171. [Google Scholar] [CrossRef] [Green Version]
- Love-Koh, J.; Peel, A.; Rejon-Parrilla, J.C.; Ennis, K.; Lovett, R.; Manca, A.; Chalkidou, A.; Wood, H.; Taylor, M. The future of precision medicine: Potential impacts for health technology assessment. Pharmacoeconomics 2018, 36, 1439–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzebonska, K.; Waligora, M. Umbrella and basket trials in oncology: Ethical challenges. BMC Med. Ethics 2019, 20, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperber, N.R.; Carpenter, J.S.; Cavallari, L.H.; Damschroder, L.J.; Cooper-DeHoff, R.M.; Denny, J.C.; Ginsburg, G.S.; Guan, Y.; Horowitz, C.R.; Levy, K.D. Challenges and strategies for implementing genomic services in diverse settings: Experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med. Genom. 2017, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Budin-Ljøsne, I.; Harris, J.R. Patient and interest organizations’ views on personalized medicine: A qualitative study. BMC Med. Ethics 2016, 17, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleidgen, S.; Marckmann, G. Re-focusing the ethical discourse on personalized medicine: A qualitative interview study with stakeholders in the German healthcare system. BMC Med. Ethics 2013, 14, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heßling, A.; Schicktanz, S. What German experts expect from individualized medicine: Problems of uncertainty and future complication in physician–patient interaction. Clin. Ethics 2012, 7, 86–93. [Google Scholar] [CrossRef]
- Schleidgen, S.; Thiersch, S.; Wuerstlein, R.; Marckmann, G. How do patients experience individualized medicine? A qualitative Interview-Based study of gene expression analyses in the treatment of breast cancer. Geburtshilfe Frauenheilkd. 2017, 77, 984. [Google Scholar] [CrossRef] [Green Version]
- Miller, F.A.; Hayeems, R.Z.; Bytautas, J.P.; Bedard, P.L.; Ernst, S.; Hirte, H.; Hotte, S.; Oza, A.; Razak, A.; Welch, S. Testing personalized medicine: Patient and physician expectations of next-generation genomic sequencing in late-stage cancer care. Eur. J. Hum. Genet. 2014, 22, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.W.; Hicks-Courant, K.; Lathan, C.S.; Garraway, L.; Park, E.R.; Weeks, J.C. Attitudes of patients with cancer about personalized medicine and somatic genetic testing. J. Oncol. Pract. 2012, 8, 329–335. [Google Scholar] [CrossRef]
- Thurner, S.; Klimek, P.; Hanel, R. Introduction to the Theory of Complex Systems; Oxford University Press: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Pavlopoulos, G.A.; Kontou, P.I.; Pavlopoulou, A.; Bouyioukos, C.; Markou, E.; Bagos, P.G. Bipartite graphs in systems biology and medicine: A survey of methods and applications. GigaScience 2018, 7, giy014. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Mehra, A.; Brass, D.J.; Labianca, G. Network analysis in the social sciences. Science 2009, 323, 892–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creswell, J.W.; Clark, V.L.P. Designing and Conducting Mixed Methods Research, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Curry, L.A.; Krumholz, H.M.; O’Cathain, A.; Clark, V.L.P.; Cherlin, E.; Bradley, E.H. Mixed methods in biomedical and health services research. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokorny, J.J.; Norman, A.; Zanesco, A.P.; Bauer-Wu, S.; Sahdra, B.K.; Saron, C.D. Network analysis for the visualization and analysis of qualitative data. Psychol. Methods 2018, 23, 169. [Google Scholar] [CrossRef] [PubMed]
- Hora, M.T. Hiring as cultural gatekeeping into occupational communities: Implications for higher education and student employability. High. Educ. 2020, 79, 307–324. [Google Scholar] [CrossRef]
- Larosa, F.; Mysiak, J. Business models for climate services: An analysis. Clim. Serv. 2020, 17, 100111. [Google Scholar] [CrossRef]
- Graham, G.; Burns, L.; Hennelly, P. Digital Transformation in the Automotive Supply Chain. In Proceedings of the 23rd Cambridge International Manufacturing Symposium, Cambridge, UK, 26–27 September 2019. [Google Scholar] [CrossRef]
- Vaismoradi, M.; Turunen, H.; Bondas, T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs. Health Sci. 2013, 15, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Gély, A.; Nourine, L.; Sadi, B. Enumeration aspects of maximal cliques and bicliques. Discret. Appl. Math. 2009, 157, 1447–1459. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, T.; Horadam, K. Finding maximal bicliques in bipartite networks using node similarity. Appl. Netw. Sci. 2019, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment of Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 13 November 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Lu, Y.; Phillips, C.A.; Langston, M.A. Biclique: An R package for maximal biclique enumeration in bipartite graphs. BMC Res. Notes 2020, 13, 88. [Google Scholar] [CrossRef] [Green Version]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. Icwsm 2009, 8, 361–362. [Google Scholar] [CrossRef]




| Theme | Code |
|---|---|
| 1. Ethics and personalized medicine (PM) (0/27) | 1.1 (Concerns about) informed consents (0/5) 1.2 Concerns about ethics approvals (0/4) 1.3 Ethical concerns (0/2) 1.4 Views on/Concerns about ethics committees (0/3) 1.5 (Issues w.r.t.) studies with ethics approvals (0/4) 1.6 (Concerns about the) importance of ethics (0/3) 1.7 Ethical issues (0/6) |
| 2. Decision-making and PM (2/29) | 2.1 Views on health technology assessment (HTA) (0/1) 2.2 (Concerns about) liquid biopsies (0/2) 2.3 Ethics and economics (0/1) 2.4 Cost concerns (0/3) 2.5 Views on health economics (1/6) 2.6 (Concerns about the) role of pharmaceutical industry (0/5) 2.7 Health economic concerns (0/7) 2.8 Lobbying (0/2) 2.9 (Concerns about) private versus public (1/2) |
| 3. Effectiveness evidence and PM (2/33) | 3.1 (Concerns about) preclinical studies (2/2) 3.2 Translational issues (0/6) 3.3 (Concerns about) knowledge gap (0/7) 3.4 Views on evidence (0/8) 3.5 Views on/Concerns about randomized controlled trials (RCTs) (0/10) |
| 4. Interpretation of PM (1/28) | 4.1 Views on hype (0/6) 4.2 Various understandings of PM (1/8) 4.3 Examples of personalized treatment (0/6) 4.4 Overdiagnosis (0/8) |
| 5. Health care (HC) systems/public health and PM (7/20) | 5.1 Views on/Concerns about genetic screening w.r.t. the public (1/3) 5.2 (Concerns w.r.t.) the Austrian context (2/7) 5.3 (Concerns about) access limitation to treatment (2/4) 5.4 Public health (1/3) 5.5 (Concerns about the) importance of preventive medicine (1/3) |
| 6. Challenges in PM (5/22) | 6.1 Views on/Concerns about progress in cancer research (1/5) 6.2 Improving understanding in society (0/3) 6.3 (Concerns about) data storage (0/2) 6.4 (Concerns about) big data collection and use (2/7) 6.5 (Concerns about) consent for data collection (2/5) |
| 7. Patient-doctor relationships and PM (4/40) | 7.1 Expertise (assessment) (1/1) 7.2 Decision-making patients’ perspective (2/0) 7.3 Concerns about patients’ participation in the decision-making process (0/6) 7.4 Shared decision-making (0/5) 7.5 (Concerns about) communication (0/8) 7.6 (Concerns about the) importance of doctor-patient relationships (0/4) 7.7 (Concerns about) incidental findings (1/5) 7.8 (Concerns about) best decision-making (0/11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelzer, I.V.; Sierawska, A.; Buyx, A.; Simon, J. A Network-Based Mixed Methods Approach to Analyze Current Perspectives on Personalized Oncological Medicine in Austria. J. Pers. Med. 2020, 10, 276. https://doi.org/10.3390/jpm10040276
Stelzer IV, Sierawska A, Buyx A, Simon J. A Network-Based Mixed Methods Approach to Analyze Current Perspectives on Personalized Oncological Medicine in Austria. Journal of Personalized Medicine. 2020; 10(4):276. https://doi.org/10.3390/jpm10040276
Chicago/Turabian StyleStelzer, Ines Viktoria, Anna Sierawska, Alena Buyx, and Judit Simon. 2020. "A Network-Based Mixed Methods Approach to Analyze Current Perspectives on Personalized Oncological Medicine in Austria" Journal of Personalized Medicine 10, no. 4: 276. https://doi.org/10.3390/jpm10040276
APA StyleStelzer, I. V., Sierawska, A., Buyx, A., & Simon, J. (2020). A Network-Based Mixed Methods Approach to Analyze Current Perspectives on Personalized Oncological Medicine in Austria. Journal of Personalized Medicine, 10(4), 276. https://doi.org/10.3390/jpm10040276