Phenotyping Type 2 Diabetes in Terms of Myocardial Insulin Resistance and Its Potential Cardiovascular Consequences: A New Strategy Based on 18F-FDG PET/CT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
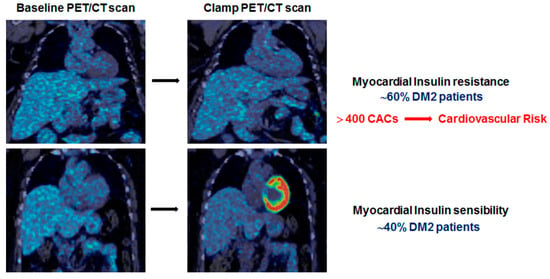
2.2. Characterization of Myocardial Insulin Resistance (mIR)
2.3. PET/CT Acquisition
2.4. Data Processing and Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godsland, I.F.; Lecamwasam, K.; Johnston, D.G. A systematic evaluation of the insulin resistance syndrome as an independent risk factor for cardiovascular disease mortality and derivation of a clinical index. Metabolism 2011, 60, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Witteles, R.M.; Fowler, M.B. Insulin-Resistant Cardiomyopathy. Clinical Evidence, Mechanisms, and Treatment Options. J. Am. Coll. Cardiol. 2008, 51, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, Z.; Zhu, Q.; Thomas, C.; Kumar, R.; Feng, H.; Dostal, D.E.; White, M.F.; Baker, K.M.; Guo, S. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38α MAPK during insulin resistance. Diabetes 2013, 62, 3887–3900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, F.; Zhao, K.; Li, J.; Xu, J.; Zhang, Y.; Liu, C.; Yang, W.; Gao, C.; Li, J.; Zhang, H.; et al. Direct Evidence that Myocardial Insulin Resistance following Myocardial Ischemia Contributes to Post-Ischemic Heart Failure. Sci. Rep. 2015, 5, 17927. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Pérez-Mañá, C.; Papaseit, E.; Menoyo, E.; Pérez, M.; Martin, S.; Bullich, S.; Rojas, S.; Herance, J.-R.; Trampal, C.; et al. Bilastine vs. hydroxyzine: Occupation of brain histamine H1-receptors evaluated by positron emission tomography in healthy volunteers. Br. J. Clin. Pharmacol. 2014, 78, 970–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voipio-Pulkki, L.M.; Nuutila, P.; Knuuti, M.J.; Ruotsalainen, U.; Haaparanta, M.; Teras, M.; Wegelius, U.; Koivisto, V.A. Heart and skeletal muscle glucose disposal in type 2 diabetic patients as determined by positron emission tomography. J. Nucl. Med. 1993, 34, 2064–2067. [Google Scholar] [PubMed]
- Feng, H.; Wang, X.; Chen, J.; Cui, J.; Gao, T.; Gao, Y.; Zeng, W. Nuclear Imaging of Glucose Metabolism: Beyond 18F-FDG. Contrast Media Mol. Imaging 2019, 2019, 7954854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, C.S.; Bailey, E.; Kumar, V.; Schwarz, S.W.; Bom, H.S.; Hatazawa, J.; Paez, D.; Orellana, P.; Louw, L.; Mut, F.; et al. Global Issues of Radiopharmaceutical Access and Availability: A Nuclear Medicine Global Initiative Project. J. Nucl. Med. 2021, 62, 422. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V.; Bacharach, S.L.; Beanlands, R.S.; Bergmann, S.R.; Delbeke, D.; Dorbala, S.; Gropler, R.J.; Knuuti, J.; Schelbert, H.R.; Travin, M.I. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J. Nucl. Cardiol. 2016, 23, 1187–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuuti, M.J.; Nuutila, P.; Ruotsalainen, U.; Saraste, M.; Häkönen, R.; Ahonen, A.; Teräs, M.; Haaparanta, M.; Wegelius, U.; Haapanen, A.; et al. Euglycemic Hyperinsulinemic Clamp and Oral Glucose Load in Stimulating Myocardial Glucose Utilization During Positron Emission Tomography. J. Nucl. Med. 1992, 33, 1255–1262. [Google Scholar] [PubMed]
- Gerber, B. Positron emission tomography using18F-fluoro-deoxyglucose and euglycaemic hyperinsulinaemic glucose clamp: Optimal criteria for the prediction of recovery of post-ischaemic left ventricular dysfunction. Results from the European Community Concerted Action Multicenter study on use of18F-fluoro-deoxyglucose Positron Emission Tomography for the Detection of Myocardial Viability. Eur. Heart J. 2001, 22, 1691–1701. [Google Scholar] [PubMed] [Green Version]
- Bax, J.J.; Visser, F.C.; Poldermans, D.; Van Lingen, A.; Elhendy, A.; Boersma, E.; Visser, C.A. Feasibility, safety and image quality of cardiac FDG studies during hyperinsulinaemic-euglycaemic clamping. Eur. J. Nucl. Med. 2002, 29, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorov, A.; Clunie, D.; Ulrich, E.; Bauer, C.; Wahle, A.; Brown, B.; Onken, M.; Riesmeier, J.; Pieper, S.; Kikinis, R.; et al. DICOM for quantitative imaging biomarker development: A standards based approach to sharing clinical data and structured PET/CT analysis results in head and neck cancer research. PeerJ 2016, 4, e2057. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.; Ferrannini, E.; Opie, L.H. Myocardial metabolism in ischemic heart disease: Basic principles and application to imaging by positron emission tomography. Prog. Cardiovasc. Dis. 1989, 32, 217–238. [Google Scholar] [CrossRef]
- Kramer, C.K.; Zinman, B.; Gross, J.L.; Canani, L.H.; Rodrigues, T.C.; Azevedo, M.J.; Retnakaran, R. Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: Systematic review and meta-analysis. BMJ 2013, 346, f1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budoff, M.J.; Raggi, P.; Beller, G.A.; Berman, D.S.; Druz, R.S.; Malik, S.; Rigolin, V.H.; Weigold, W.G.; Soman, P. Noninvasive Cardiovascular Risk Assessment of the Asymptomatic Diabetic Patient: The Imaging Council of the American College of Cardiology. JACC Cardiovasc. Imaging 2016, 9, 176–192. [Google Scholar] [CrossRef] [PubMed]


| N = 47 | |
|---|---|
| Age (years) | 67 ± 7 |
| Gender (M/F) | 24/23 |
| BMI (Kg/m2) | 30.76 [28.74–35.11] |
| Waist (cm) | 108 [99–117] |
| HOMA-IR | 4.76 [3.22–6.58] |
| HbA1c (%) | 7.30 [6.60–7.50] |
| HbA1c (mmol/mol) | 56 [51–58] |
| Total Cholesterol (mmol/L) | 4.24 [3.83–5.07] |
| HDL-C (mmol/L) | 1.19 [1.01–1.35] |
| LDL-C (mmol/L) | 2.38 [2.02–2.97] |
| Triglycerides (mmol/L) | 1.26 [0.94–1.99] |
| CACs > 400 AU (% patients) | 42.5 |
| mRD (HU) | 36.05 [28.99–41.16] |
| ΔSUV | 1.56 [0.55–5.82] |
| mIS (N = 21) | mIR (N = 26) | p | |
|---|---|---|---|
| Age (years) | 70 ± 8 | 66 ± 6 | 0.18 |
| Gender (M/F) | 11/10 | 13/13 | 0.99 |
| BMI (Kg/m2) | 30.46 [27.83–37.22] | 31.16 [29.27–34.87] | 0.50 |
| Waist (cm) | 107 [99–117] | 109 [100–115] | 0.82 |
| HOMA-IR | 3.88 [3.21–5.26] | 5.51 [4.39–9.98] | 0.03 |
| Hb1Ac (%) | 7.05 [6.48–7.40] | 7.40 [6.80–7.70] | 0.35 |
| HbA1c (mmol/mol) | 54 [47–57] | 57 [51–61] | 0.35 |
| Total Cholesterol (mmol/L) | 4.17 [3.65–4.43] | 4.56 [4.01–5.36] | 0.17 |
| HDL-C (mmol/L) | 1.24 [0.98–1.34] | 1.11 [0.98–1.29] | 0.30 |
| LDL-C (mmol/L) | 2.17 [1.97–2.64] | 2.72 [2.12–3.16] | 0.30 |
| Triglycerides (mmol/L) | 1.23 [0.82–1.87] | 1.4 [1.29–2.19] | 0.25 |
| CACs > 400 AU (% patients) | 29 | 52 | <0.01 |
| mRD (HU) | 30.82 [21.48–38.02] | 38.95 [33.81–44.06] | <0.01 |
| ΔSUV | 6.165 [5.625–7.615] | 0.7750 [0.2675–1.468] | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herance, J.R.; Simó, R.; Velasquez, M.A.; Paun, B.; García-Leon, D.; Aparicio, C.; Marés, R.; Simó-Servat, O.; Castell-Conesa, J.; Hernández, C.; et al. Phenotyping Type 2 Diabetes in Terms of Myocardial Insulin Resistance and Its Potential Cardiovascular Consequences: A New Strategy Based on 18F-FDG PET/CT. J. Pers. Med. 2022, 12, 30. https://doi.org/10.3390/jpm12010030
Herance JR, Simó R, Velasquez MA, Paun B, García-Leon D, Aparicio C, Marés R, Simó-Servat O, Castell-Conesa J, Hernández C, et al. Phenotyping Type 2 Diabetes in Terms of Myocardial Insulin Resistance and Its Potential Cardiovascular Consequences: A New Strategy Based on 18F-FDG PET/CT. Journal of Personalized Medicine. 2022; 12(1):30. https://doi.org/10.3390/jpm12010030
Chicago/Turabian StyleHerance, José Raul, Rafael Simó, Mayra Alejandra Velasquez, Bruno Paun, Daniel García-Leon, Carolina Aparicio, Roso Marés, Olga Simó-Servat, Joan Castell-Conesa, Cristina Hernández, and et al. 2022. "Phenotyping Type 2 Diabetes in Terms of Myocardial Insulin Resistance and Its Potential Cardiovascular Consequences: A New Strategy Based on 18F-FDG PET/CT" Journal of Personalized Medicine 12, no. 1: 30. https://doi.org/10.3390/jpm12010030
APA StyleHerance, J. R., Simó, R., Velasquez, M. A., Paun, B., García-Leon, D., Aparicio, C., Marés, R., Simó-Servat, O., Castell-Conesa, J., Hernández, C., & Aguadé-Bruix, S. (2022). Phenotyping Type 2 Diabetes in Terms of Myocardial Insulin Resistance and Its Potential Cardiovascular Consequences: A New Strategy Based on 18F-FDG PET/CT. Journal of Personalized Medicine, 12(1), 30. https://doi.org/10.3390/jpm12010030