Combined versus Single Perforator Propeller Flaps for Reconstruction of Large Soft Tissue Defects: A Retrospective Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
Flap Harvesting
2.3. Statistical Analysis
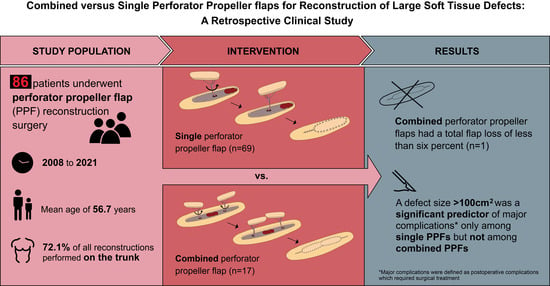
3. Results
3.1. Overall Sample Characteristics
3.2. Comparison of Single PPFs and Combined PPFs
3.3. Univariable Binary Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumann, D.P.; Butler, C.E. Soft tissue coverage in abdominal wall reconstruction. Surg. Clin. N. Am. 2013, 93, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Falkner, F.; Thomas, B.; Haug, V.; Nagel, S.S.; Vollbach, F.H.; Kneser, U.; Bigdeli, A.K. Comparison of pedicled versus free flaps for reconstruction of extensive deep sternal wound defects following cardiac surgery: A retrospective study. Microsurgery 2021, 41, 309–318. [Google Scholar] [CrossRef]
- Xiong, L.; Gazyakan, E.; Kremer, T.; Hernekamp, F.J.; Harhaus, L.; Saint-Cyr, M.; Kneser, U.; Hirche, C. Free flaps for reconstruction of soft tissue defects in lower extremity: A meta-analysis on microsurgical outcome and safety. Microsurgery 2016, 36, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, A.K.; Thomas, B.; Falkner, F.; Radu, C.A.; Gazyakan, E.; Kneser, U. Microsurgical reconstruction of extensive lower extremity defects with the conjoined parascapular and latissimus dorsi free flap. Microsurgery 2020, 40, 639–648. [Google Scholar] [CrossRef]
- Beier, J.P.; Horch, R.E.; Kneser, U. Bilateral pre-expanded free TFL flaps for reconstruction of severe thoracic scar contractures in an 8-year-old girl. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Wanjala, N.F.; Dan, K. Local/regional flaps for extensive abdominal wall defects: Case series. Int. J. Surg. Case Rep. 2020, 74, 10–14. [Google Scholar] [CrossRef]
- Ebehr, B.; Wagner, J.M.; Ewallner, C.; Eharati, K.; Elehnhardt, M.; Daigeler, A. Reconstructive Options for Oncologic Posterior Trunk Defects: A Review. Front. Oncol. 2016, 6, 51. [Google Scholar] [CrossRef]
- Hernekamp, J.-F.; Cordts, T.; Kremer, T.; Kneser, U. Perforator-Based Flaps for Defect Reconstruction of the Posterior Trunk. Ann. Plast. Surg. 2020, 86, 72–77. [Google Scholar] [CrossRef]
- Hallock, G.G. The Propeller Flap Version of the Adductor Muscle Perforator Flap for Coverage of Ischial or Trochanteric Pressure Sores. Ann. Plast. Surg. 2006, 56, 540–542. [Google Scholar] [CrossRef]
- Yang, C.-H.; Kuo, Y.-R.; Jeng, S.-F.; Lin, P.-Y. An Ideal Method for Pressure Sore Reconstruction: A freestyle perforator-based flap. Ann. Plast. Surg. 2011, 66, 179–184. [Google Scholar] [CrossRef]
- Pignatti, M.; Ogawa, R.; Hallock, G.G.; Mateev, M.; Georgescu, A.V.; Balakrishnan, G.; Ono, S.; Cubison, T.C.S.; D’arpa, S.; Koshima, I.; et al. The “Tokyo” Consensus on Propeller Flaps. Plast. Reconstr. Surg. 2011, 127, 716–722. [Google Scholar] [CrossRef]
- Yu, S.; Zang, M.; Xu, L.; Zhao, Z.; Zhang, X.; Zhu, S.; Chen, B.; Ding, Q.; Liu, Y. Perforator Propeller Flap for Oncologic Reconstruction of Soft Tissue Defects in Trunk and Extremities. Ann. Plast. Surg. 2016, 77, 456–463. [Google Scholar] [CrossRef]
- Ellabban, M.A.; Wyckman, A.; Abdelrahman, I.; Steinvall, I.; Elmasry, M. Dual Reconstruction of Lumbar and Gluteal Defects with Freestyle Propeller Flap and Muscle Flap. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3376. [Google Scholar] [CrossRef]
- Schaffer, C.; Guillier, D.; Raffoul, W.; di Summa, P.G. Lumbar Perforator Flaps for Coverage of Extensive Defects With Osteomyelitis. Ann. Plast. Surg. 2021, 86, 67–71. [Google Scholar] [CrossRef]
- Falinower, H.; Herlin, C.; Laloze, J.; Bodin, F.; Kerfant, N.; Chaput, B. Use of the Propeller Lumbar Perforator Flap: A Series of 32 Cases. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2522. [Google Scholar] [CrossRef]
- Boissière, F.; Luca-Pozner, V.; Vaysse, C.; Kerfant, N.; Herlin, P.C.; Chaput, P.B. The SCIP propeller flap: Versatility for reconstruction of locoregional defect. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1121–1128. [Google Scholar] [CrossRef]
- Park, S.W.; Oh, T.S.; Eom, J.S.; Sun, Y.C.; Suh, H.S.; Hong, J.P. Freestyle Multiple Propeller Flap Reconstruction (Jigsaw Puzzle Approach) for Complicated Back Defects. J. Reconstr. Microsurg. 2015, 31, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Haug, V.; Falkner, F.; Arras, C.; Nagel, S.S.; Boecker, A.; Schmidt, V.J.; Kneser, U.; Bigdeli, A.K. A single-center retrospective comparison of Duplex ultrasonography versus audible Doppler regarding anterolateral thigh perforator flap harvest and operative times. Microsurgery, 2021; published online ahead of print. [Google Scholar] [CrossRef]
- Daigeler, A.; Schubert, C.; Hirsch, T.; Behr, B.; Lehnhardt, M. Colour duplex sonography and “Power-Duplex” in Perforator Surgery—Improvement of patients safety by efficient planning. Handchir. Mikrochir. Plast. Chir. 2018, 50, 101–110. [Google Scholar] [CrossRef]
- Wong, C.-H.; Cui, F.; Tan, B.-K.; Liu, Z.; Lee, H.-P.; Lu, C.; Foo, C.-L.; Song, C. Nonlinear Finite Element Simulations to Elucidate the Determinants of Perforator Patency in Propeller Flaps. Ann. Plast. Surg. 2007, 59, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, G.; Anicic, S.; Formaggia, L. Mathematical explanation of the buckling of the vessels after twisting of the microanastomosis. Microsurgery 2006, 26, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Topalan, M.; Bilgin, S.S.; Ip, W.; Chow, S. Effect of torsion on microarterial anastomosis patency. Microsurgery 2003, 23, 56–59. [Google Scholar] [CrossRef]
- Bigdeli, A.K.; Thomas, B.; Falkner, F.; Gazyakan, E.; Hirche, C.; Kneser, U. The Impact of Indocyanine-Green Fluorescence Angiography on Intraoperative Decision-Making and Postoperative Outcome in Free Flap Surgery. J. Reconstr. Microsurg. 2020, 36, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Kuo, P.; Kuo, S.C.H.; Chien, P.; Hsieh, C. Risk factors associated with postoperative complications of free anterolateral thigh flap placement in patients with head and neck cancer: Analysis of propensity score-matched cohorts. Microsurgery 2020, 40, 538–544. [Google Scholar] [CrossRef]
- Peng, P.; Dong, Z.; Wei, J.; Liu, L.; Luo, Z.; Zheng, L. Risk factors related to the partial necrosis of the posterior tibial artery perforator-plus fasciocutaneous flap. Eur. J. Trauma Emerg. Surg. 2021. published online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jakubietz, R.G.; Schmidt, K.; Bernuth, S.; Meffert, R.H.; Jakubietz, M.G. Evaluation of the Intraoperative Blood Flow of Pedicled Perforator Flaps Using Indocyanine Green-fluorescence Angiography. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2462. [Google Scholar] [CrossRef]
- Kneser, U.; Beier, J.P.; Schmitz, M.; Arkudas, A.; Dragu, A.; Schmidt, V.J.; Kremer, T.; Horch, R.E. Zonal perfusion patterns in pedicled free-style perforator flaps. J. Plast. Reconstr. Aesthetic Surg. 2013, 67, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Henn, D.; Wähmann, M.S.T.; Horsch, M.; Hetjens, S.; Kremer, T.; Gazyakan, E.; Hirche, C.; Schmidt, V.J.; Germann, G.; Kneser, U. One-Stage versus Two-Stage Arteriovenous Loop Reconstructions: An Experience on 103 Cases from a Single Center. Plast. Reconstr. Surg. 2019, 143, 912–924. [Google Scholar] [CrossRef]
- Farquhar, D.R.; Masood, M.M.; Pappa, A.K.; Patel, S.N.; Hackman, A.T.G. Predictors of Adverse Outcomes in Free Flap Reconstruction: A Single-Institution Experience. Otolaryngol. Neck Surg. 2018, 159, 973–980. [Google Scholar] [CrossRef]
- Le Nobel, G.J.; Higgins, K.M.; Enepekides, D.J. Predictors of complications of free flap reconstruction in head and neck surgery: Analysis of 304 free flap reconstruction procedures. Laryngoscope 2012, 122, 1014–1019. [Google Scholar] [CrossRef]
- Classen, D.A.; Ward, H. Complications in a Consecutive Series of 250 Free Flap Operations. Ann. Plast. Surg. 2006, 56, 557–561. [Google Scholar] [CrossRef]
- Guo, F.; Shashikiran, T.; Chen, X.; Yang, L.; Liu, X.; Song, L. Clinical features and risk factor analysis for lower extremity deep venous thrombosis in Chinese neurosurgical patients. J. Neurosci. Rural. Pract. 2015, 6, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Brogan, E.; Langdon, C.; Brookes, K.; Budgeon, C.; Blacker, D. Respiratory Infections in Acute Stroke: Nasogastric Tubes and Immobility are Stronger Predictors than Dysphagia. Dysphagia 2014, 29, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-L.; Lee, W.-R.; Yeh, C.-C.; Shih, C.-C.; Chen, T.-L.; Liao, C.-C. Adverse Outcomes after Major Surgery in Patients with Pressure Ulcer: A Nationwide Population-Based Retrospective Cohort Study. PLoS ONE 2015, 10, e0127731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitse, J.; Bekara, F.; Bertheuil, N.; Sinna, R.; Chaput, B.; Herlin, C. Perforator-based propeller flaps reliability in upper extremity soft tissue reconstruction: A systematic review. J. Hand Surg. Eur. Vol. 2016, 42, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Yoshimatsu, H.; Koshima, I. Reconstruction of Anterolateral Thigh Defects Using Perforator-Based Propeller Flaps. Ann. Plast. Surg. 2017, 79, 385–389. [Google Scholar] [CrossRef]
- Arco, G.; Horch, R.E.; Arkudas, A.; Dragu, A.; Bach, A.D.; Kneser, U. Double pedicled perforator flap to close flank defects: An alternative for closure of a large lumbar defect after basalioma excision--A case report and review of the literature. Ann. Plast. Surg. 2009, 63, 422–424. [Google Scholar] [CrossRef]
- Scaglioni, M.F.; Grufman, V.; Meroni, M.; Fritsche, E. Soft tissue coverage of a total gluteal defect with a combination of perforator-based flaps: A case report. Microsurgery 2020, 40, 797–801. [Google Scholar] [CrossRef]


| Title | Author | Year | No. of Patients | Body Region |
|---|---|---|---|---|
| Dual Reconstruction of Lumbar and Gluteal Defects with Freestyle Propeller Flap and Muscle Flap | Ellabban et al. [13] | 2021 | 18 | Trunk/Gluteal |
| Lumbar Perforator Flaps for Coverage of Extensive Defects With Osteomyelitis | Schaffer et al. [14] | 2021 | 7 | Trunk |
| Perforator-Based Flaps for Defect Reconstruction of the Posterior Trunk | Hernekamp et al. [9] | 2021 | 36 | Trunk |
| Use of the Propeller Lumbar Perforator Flap: A Series of 32 Cases | Falinower et al. [15] | 2020 | 31 | Trunk |
| The SCIP propeller flap: Versatility for reconstruction of locoregional defect | Boissière et al. [16] | 2019 | 56 | Trunk |
| Freestyle multiple propeller flap reconstruction (jigsaw puzzle approach) for complicated back defects | Park et al. [17] | 2015 | 18 | Trunk |
| Characteristic | Total | Single PPF | Combined PPF | p-Value |
|---|---|---|---|---|
| No. of patients (%) | 86 | 69 (80.2) | 17 (19.8) | |
| Combined PPF, No. (%) | 17 (19.8) | |||
| Double PPF | 11 (64.7) | |||
| PPF plus regional flap | 6 (35.3) | |||
| Sex, No. (%) | 0.79 | |||
| Female | 38 (44.2) | 30 (43.5) | 8 (47.1) | |
| Male | 48 (55.8) | 39 (56.5) | 9 (52.9) | |
| Mean age [years] (SD, range) | 56.7 (19.7, 4−88) | 55.7 (20.0, 4−86) | 60.8 (18.6, 21−88) | 0.34 |
| Risk factors 1 present, No. (%) | 29 (33.7) | 19 (27.5) | 10 (58.8) | 0.02 |
| Defect etiology (%) | ||||
| Burn injury | 1 (1.2) | 1 (1.4) | 0 (0.0) | 0.36 |
| Pressure ulcer | 13 (15.1) | 10 (14.5) | 3 (17.6) | 0.72 |
| Infection | 8 (9.3) | 6 (8.7) | 2 (11.8) | 0.65 |
| Trauma | 6 (7.0) | 4 (5.8) | 2 (11.8) | 0.39 |
| Tumor | 44 (51.2) | 34 (49.3) | 10 (58.8) | 0.59 |
| Other | 14 (16.3) | 14 (20.3) | 0 (0.0) | |
| Defect size in [cm2] (SD, range) | 117.8 (88.6, 12−504) | 103.0 (73.5, 12−450) | 178.2 (73.8, 25−504) | <0.01 |
| PPF size [cm2] (SD, range) | 137.3 (85.1, 24−532) | 132.8 (88.0, 24−532) | 155.2 (71.6, 32−341) | 0.10 |
| Flap location (%) | ||||
| Trunk | 62 (72.1) | 47 (68.1) | 15 (88.2) | 0.13 |
| Lower limb | 18 (20.9) | 16 (23.2) | 2 (11.8) | 0.50 |
| Upper limb | 6 (7.0) | 6 (8.7) | 0 (0.0) | 0.34 |
| Operation time [min] (SD, range) | 177.6 (68.0, 80−480) | 164.0 (59.0, 80−440) | 232.9 (75.6, 127−480) | <0.01 |
| Flap rotation [degree] (SD, range) | 149.9 (35.0, 50−180) | 147.1 (37.1, 50−180) | 156.4 (29.8, 90−180) | 0.53 |
| Number of surgeries 2 (SD, range) | 1.7 (1.4, 1−8) | 1.5 (1.1, 1−7) | 2.3 (2.0, 1−8) | 0.16 |
| Major complications 3 (%) | 27 (31.4) | 22 (31.9) | 5 (29.4) | 0.32 |
| Flap loss (%) | ||||
| Partial | 5 (7.2) | 5 (7.2) | 0 (0.0) | 0.56 |
| Complete | 5 (5.8) | 4 (5.8) | 1 (5.9) | 0.99 |
| Total hospitalization [days] (SD, range) | 34.7 (15.7, 14−84) | 32.5 (13.6, 15−61) | 39.7 (19.7, 14−84) | 0.31 |
| Characteristics | Total | Single PPF | Combined PPF |
|---|---|---|---|
| No. of PPFs (%) | 97 | 69 (71.1) | 28 (28.9) |
| PPF type, No. (%) | |||
| Adductor perforator | 5 (5.2) | 3 (4.3) | 2 (7.1) |
| ALT | 5 (5.2) | 4 (5.8) | 1 (3.6) |
| ATA | 3 (3.0) | 3 (4.3) | 0 (0.0) |
| AIA | 3 (3.0) | 3 (4.3) | 0 (0.0) |
| PTA | 5 (5.2) | 5 (7.2) | 0 (0.0) |
| Brachial artery | 4 (4.1) | 4 (5.8) | 0 (0.0) |
| DICAP | 7 (7.3) | 4 (5.8) | 3 (10.7) |
| IGAP | 15 (15.5) | 9 (13.0) | 6 (21.4) |
| LICAP | 3 (3.0) | 2 (2.9) | 1 (3.6) |
| Radial artery | 3 (3.0) | 3 (4.3) | 0 (0.0) |
| SGAP | 14 (14.4) | 8 (11.6) | 6 (21.4) |
| Lateral genicular artery | 1 (1.0) | 1 (1.4) | 0 (0.0) |
| LAP | 18 (18.6) | 10 (14.5) | 8 (28.6) |
| Posterior thigh perforator | 2 (2.0) | 2 (2.9) | 0 (0.0) |
| Profound femoral artery | 3 (3.0) | 3 (4.3) | 0 (0.0) |
| Pudendal artery | 2 (2.0) | 1 (1.4) | 1 (3.6) |
| Thoracoacromial artery | 2 (2.0) | 2 (2.9) | 0 (0.0) |
| Trapezius perforator | 2 (2.0) | 2 (2.9) | 0 (0.0) |
| Total PPF (n = 86) | Single PPF (n = 69) | Combined PPF (n = 17) | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | Odds Ratio | Odds Ratio | ||||
| Characteristics | (95% CI) | p-Value | (95% CI) | p-Value | (95% CI) | p-Value |
| Intervention | ||||||
| Single PPF | 1 [Reference] | |||||
| Combined PPF | 0.95 (0.27−2.93) | 0.29 | ||||
| Operation time (min) | 1.00 (1.00−1.01) | 0.25 | 1.00 (0.99−1.01) | 0.80 | 1.01 (1.00−1.04) | 0.33 |
| PPF size (cm2) | 1.00 (0.99−1.01) | 0.15 | 1.00 (1.00−1.01) | 0.50 | 1.01 (1.00−1.03) | 0.11 |
| Defect size (cm2) | ||||||
| <50 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| 50−99 | 3.97 (0.80−29.48) | 0.17 | 6.85 (1.00−137.99) | 0.13 | 1.00 (0.01−69.47) | 0.99 |
| 100−199 | 4.25 (0.97−29.89) | 0.08 | 10.00 (1.63−194.65) | 0.05 | 0.17 (0.01−6.53) | 0.58 |
| >200 | 8.50 (1.51−70.24) | <0.01 | 32.00 (3.05−850.50) | 0.01 | 0.50 (0.01−17.47) | 0.99 |
| Defect size (cm2) | ||||||
| <100 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| >100 | 1.88 (0.74−4.91) | 0.18 | 2.82 (1.01−8.36) | 0.05 | 0.30 (0.02−3.37) | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigdeli, A.K.; Didzun, O.; Thomas, B.; Harhaus, L.; Gazyakan, E.; Horch, R.E.; Kneser, U. Combined versus Single Perforator Propeller Flaps for Reconstruction of Large Soft Tissue Defects: A Retrospective Clinical Study. J. Pers. Med. 2022, 12, 41. https://doi.org/10.3390/jpm12010041
Bigdeli AK, Didzun O, Thomas B, Harhaus L, Gazyakan E, Horch RE, Kneser U. Combined versus Single Perforator Propeller Flaps for Reconstruction of Large Soft Tissue Defects: A Retrospective Clinical Study. Journal of Personalized Medicine. 2022; 12(1):41. https://doi.org/10.3390/jpm12010041
Chicago/Turabian StyleBigdeli, Amir K., Oliver Didzun, Benjamin Thomas, Leila Harhaus, Emre Gazyakan, Raymund E. Horch, and Ulrich Kneser. 2022. "Combined versus Single Perforator Propeller Flaps for Reconstruction of Large Soft Tissue Defects: A Retrospective Clinical Study" Journal of Personalized Medicine 12, no. 1: 41. https://doi.org/10.3390/jpm12010041
APA StyleBigdeli, A. K., Didzun, O., Thomas, B., Harhaus, L., Gazyakan, E., Horch, R. E., & Kneser, U. (2022). Combined versus Single Perforator Propeller Flaps for Reconstruction of Large Soft Tissue Defects: A Retrospective Clinical Study. Journal of Personalized Medicine, 12(1), 41. https://doi.org/10.3390/jpm12010041