A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. HPSC Culture and Generation of hPSC-CMs
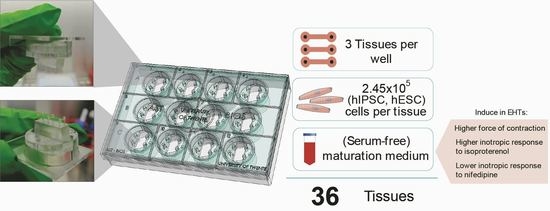
2.2. Fabrication of Engineered Heart Tissues (EHT) Platform
2.3. Conditioning of CMs and EHT Formation
2.4. Functional Analysis
2.5. Immunostaining, Histology, and Imaging
2.6. Gene Expression
2.7. Statistics
3. Results
3.1. Fabrication of the Platform
3.2. Maturation Medium Improves Tissue Formation and Induces an Increase in Contraction Force
3.3. EHTs in Maturation Medium Respond to Positive and Negative Inotropic Agents
3.4. Maturation Medium Improves Tissue Morphology and Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528, Erratum in Circulation 2020, 141, e33. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Overcoming the Declining Trends in Innovation and Investment in Cardiovascular Therapeutics. JACC Basic Transl. Sci. 2017, 2, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Chow, Y.; Esteban, M.A.; Pei, D.; Tse, H.-F. Future perspective of induced pluripotent stem cells for diagnosis, drug screening and treatment of human diseases. Thromb. Haemost. 2010, 104, 39–44. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passier, R.; Orlova, V.; Mummery, C. Complex Tissue and Disease Modeling using hiPSCs. Cell Stem Cell 2016, 18, 309–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Pabon, L.; Murry, C.E. Engineering Adolescence. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Kolanowski, T.J.; Antos, C.L.; Guan, K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. Int. J. Cardiol. 2017, 241, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.; Eder, A.; Bönstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schwörer, A.; Uebeler, J.; Eschenhagen, T. Development of a Drug Screening Platform Based on Engineered Heart Tissue. Circ. Res. 2010, 107, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Eschenhagen, T.; Eder, A.; Vollert, I.; Hansen, A. Physiological aspects of cardiac tissue engineering. Am. J. Physiol. Circ. Physiol. 2012, 303, H133–H143. [Google Scholar] [CrossRef] [Green Version]
- Nunes, S.S.; Miklas, J.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Massé, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods 2013, 10, 781–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Brenière, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schulze, T.; et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Rep. 2016, 7, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Valk, J.; Brunner, D.; De Smet, K.; Svenningsen, Å.F.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.L.; et al. Optimization of chemically defined cell culture media—Replacing fetal bovine serum in mammalian in vitro methods. Toxicol. In Vitro 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dambrot, C.; Braam, S.R.; Tertoolen, L.G.J.; Birket, M.; Atsma, D.E.; Mummery, C.L. Serum supplemented culture medium masks hypertrophic phenotypes in human pluripotent stem cell derived cardiomyocytes. J. Cell. Mol. Med. 2014, 18, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Birket, M.; Ribeiro, M.C.; Kosmidis, G.; Ward, D.; Leitoguinho, A.R.; van de Pol, V.; Dambrot, C.; Devalla, H.D.; Davis, R.; Mastroberardino, P.G.; et al. Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function. Cell Rep. 2015, 13, 733–745. [Google Scholar] [CrossRef] [Green Version]
- Schaaf, S.; Shibamiya, A.; Mewe, M.; Eder, A.; Stoehr, A.; Hirt, M.N.; Rau, T.; Zimmermann, W.-H.; Conradi, L.; Eschenhagen, T.; et al. Human Engineered Heart Tissue as a Versatile Tool in Basic Research and Preclinical Toxicology. PLoS ONE 2011, 6, e26397. [Google Scholar] [CrossRef]
- Cashman, T.; Josowitz, R.; Gelb, B.D.; Li, R.A.; Dubois, N.C.; Costa, K.D. Construction of Defined Human Engineered Cardiac Tissues to Study Mechanisms of Cardiac Cell Therapy. J. Vis. Exp. 2016, 2016, e53447. [Google Scholar] [CrossRef]
- A Elliott, D.; Braam, S.R.; Koutsis, K.; Ng, E.S.; Jenny, R.; Lagerqvist, E.L.; Biben, C.; Hatzistavrou, T.; E Hirst, C.; Yu, Q.C.; et al. NKX2-5eGFP/w hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat. Methods 2011, 8, 1037–1040. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Slaats, R.H.; Schwach, V.; Rivera-Arbelaez, J.M.; Tertoolen, L.G.; van Meer, B.J.; Molenaar, R.; Mummery, C.L.; Claessens, M.M.; Passier, R. A cardiomyocyte show of force: A fluorescent alpha-actinin reporter line sheds light on human cardiomyocyte contractility versus substrate stiffness. J. Mol. Cell. Cardiol. 2020, 141, 54–64. [Google Scholar] [CrossRef]
- Ng, E.S.; Davis, R.; Stanley, E.G.; Elefanty, A. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat. Protoc. 2008, 3, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Breckwoldt, K.; Letuffe-Brenière, D.; Mannhardt, I.; Schulze, T.; Ulmer, B.; Werner, T.; Benzin, A.; Klampe, B.; Reinsch, M.; Laufer, S.; et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 2017, 12, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Serrao, G.W.; Turnbull, I.C.; Ancukiewicz, D.; Kim, D.E.; Kao, E.; Cashman, T.; Hadri, L.; Hajjar, R.J.; Costa, K.D. Myocyte-Depleted Engineered Cardiac Tissues Support Therapeutic Potential of Mesenchymal Stem Cells. Tissue Eng. Part A 2012, 18, 1322–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudou, T.; Legant, W.R.; Mu, A.; Borochin, M.A.; Thavandiran, N.; Radisic, M.; Zandstra, P.W.; Epstein, J.A.; Margulies, K.B.; Chen, C. A Microfabricated Platform to Measure and Manipulate the Mechanics of Engineered Cardiac Microtissues. Tissue Eng. Part A 2012, 18, 910–919. [Google Scholar] [CrossRef]
- Rodriguez, M.L.; Graham, B.T.; Pabon, L.M.; Han, S.J.; Murry, C.E.; Sniadecki, N.J. Measuring the Contractile Forces of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes With Arrays of Microposts. J. Biomech. Eng. 2014, 136, 051005–05100510. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Kunihiro, T.; Ando, T.; Kobayashi, S.; Matsui, E.; Yada, H.; Kanda, Y.; Kurokawa, J.; Furukawa, T. Image-based evaluation of contraction–relaxation kinetics of human-induced pluripotent stem cell-derived cardiomyocytes: Correlation and complementarity with extracellular electrophysiology. J. Mol. Cell. Cardiol. 2014, 77, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef]
- Sala, L.; Van Meer, B.J.; Tertoolen, L.G.; Bakkers, J.; Bellin, M.; Davis, R.P.; Denning, C.; Dieben, M.A.E.; Eschenhagen, T.; Giacomelli, E.; et al. Versatile open software to quantify cardiomyocyte and cardiac muscle contraction in vitro and in vivo. bioRxiv 2017, 160754. [Google Scholar] [CrossRef] [Green Version]
- Pamies, D.; Bal-Price, A.; Chesne, C.; Coecke, S.; Dinnyes, A.; Eskes, C.; Grillari, R.; Gstraunthaler, G.; Hartung, T.; Jennings, P.; et al. Advanced Good Cell Culture Practice for human primary, stem cell-derived and organoid models as well as microphysiological systems. ALTEX 2018, 35, 353–378. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Didié, M.; Münzel, F.; Schubert, P.; Schneiderbanger, K.; Zimmermann, W.H. 3D engineered heart tissue for replacement therapy. Basic Res. Cardiol. Suppl. 2002, 97, 146–152. [Google Scholar] [CrossRef]
- Schocken, D.; Stohlman, J.; Vicente, J.; Chan, D.; Patel, D.; Matta, M.K.; Patel, V.; Brock, M.; Millard, D.; Ross, J.; et al. Comparative analysis of media effects on human induced pluripotent stem cell-derived cardiomyocytes in proarrhythmia risk assessment. J. Pharmacol. Toxicol. Methods 2018, 90, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, J.P.; Mikos, A.G.; Bronzino, J.D. Tissue Engineering, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Franke, J.; Abs, V.; Zizzadoro, C.; Abraham, G. Comparative study of the effects of fetal bovine serum versus horse serum on growth and differentiation of primary equine bronchial fibroblasts. BMC Vet. Res. 2014, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito, H.; Melnychenko, I.; Didié, M.; Schneiderbanger, K.; Schubert, P.; Rosenkranz, S.; Eschenhagen, T.; Zimmermann, W.-H. Optimizing Engineered Heart Tissue for Therapeutic Applications as Surrogate Heart Muscle. Circulation 2006, 114, I-72–I-78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.C.; Tertoolen, L.G.; Guadix, J.A.; Bellin, M.; Kosmidis, G.; D’Aniello, C.; Monshouwer-Kloots, J.; Goumans, M.-J.; Wang, Y.-L.; Feinberg, A.W.; et al. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro—Correlation between contraction force and electrophysiology. Biomaterials 2015, 51, 138–150. [Google Scholar] [CrossRef]
- Bellin, M.; Casini, S.; Davis, R.; D’Aniello, C.; Haas, J.; Oostwaard, D.W.-V.; Tertoolen, L.G.J.; Jung, C.B.; Elliott, D.; Welling, A.; et al. Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long-QT syndrome. EMBO J. 2013, 32, 3161–3175. [Google Scholar] [CrossRef] [PubMed]
- Eder, A.; Vollert, I.; Hansen, A.; Eschenhagen, T. Human engineered heart tissue as a model system for drug testing. Adv. Drug Deliv. Rev. 2016, 96, 214–224. [Google Scholar] [CrossRef]
- Maier, L.S.; Schwan, C.; Schillinger, W.; Minami, K.; Schütt, U.; Pieske, B. Gingerol, isoproterenol and ouabain normalize impaired post-rest behavior but not force-frequency relation in failing human myocardium. Cardiovasc. Res. 2000, 45, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.; Nguyen, W.; Nguyenton, B.; Ratchada, P.; Page, G.; Miller, P.E.; Ghetti, A.; Abi-Gerges, N. Adult Human Primary Cardiomyocyte-Based Model for the Simultaneous Prediction of Drug-Induced Inotropic and Pro-arrhythmia Risk. Front. Physiol. 2017, 8, 1073. [Google Scholar] [CrossRef] [Green Version]
- Birket, M.; Casini, S.; Kosmidis, G.; Elliott, D.; Gerencser, A.A.; Baartscheer, A.; Schumacher, C.; Mastroberardino, P.G.; Elefanty, A.; Stanley, E.G.; et al. PGC-1α and Reactive Oxygen Species Regulate Human Embryonic Stem Cell-Derived Cardiomyocyte Function. Stem Cell Rep. 2013, 1, 560–574. [Google Scholar] [CrossRef] [Green Version]
- Polonchuk, L.; Surija, L.; Lee, M.H.; Sharma, P.; Ming, C.L.C.; Richter, F.; Ben-Sefer, E.; Rad, M.A.; Sarmast, H.M.S.; Al Shamery, W.; et al. Towards engineering heart tissues from bioprinted cardiac spheroids. Biofabrication 2021, 13, 045009. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.M.; Mummery, C.L.; Bellin, M. Engineered models of the human heart: Directions and challenges. Stem Cell Rep. 2021, 16, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, E.; Bellin, M.; Sala, L.; van Meer, B.J.; Tertoolen, L.G.J.; Orlova, V.V.; Mummery, C.L. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 2017, 144, 1008–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.C.; Rivera-Arbeláez, J.M.; Cofiño-Fabres, C.; Schwach, V.; Slaats, R.H.; ten Den, S.A.; Vermeul, K.; van den Berg, A.; Pérez-Pomares, J.M.; Segerink, L.I.; et al. A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues. J. Pers. Med. 2022, 12, 214. https://doi.org/10.3390/jpm12020214
Ribeiro MC, Rivera-Arbeláez JM, Cofiño-Fabres C, Schwach V, Slaats RH, ten Den SA, Vermeul K, van den Berg A, Pérez-Pomares JM, Segerink LI, et al. A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues. Journal of Personalized Medicine. 2022; 12(2):214. https://doi.org/10.3390/jpm12020214
Chicago/Turabian StyleRibeiro, Marcelo C., José M. Rivera-Arbeláez, Carla Cofiño-Fabres, Verena Schwach, Rolf H. Slaats, Simone A. ten Den, Kim Vermeul, Albert van den Berg, José M Pérez-Pomares, Loes I. Segerink, and et al. 2022. "A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues" Journal of Personalized Medicine 12, no. 2: 214. https://doi.org/10.3390/jpm12020214
APA StyleRibeiro, M. C., Rivera-Arbeláez, J. M., Cofiño-Fabres, C., Schwach, V., Slaats, R. H., ten Den, S. A., Vermeul, K., van den Berg, A., Pérez-Pomares, J. M., Segerink, L. I., Guadix, J. A., & Passier, R. (2022). A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues. Journal of Personalized Medicine, 12(2), 214. https://doi.org/10.3390/jpm12020214