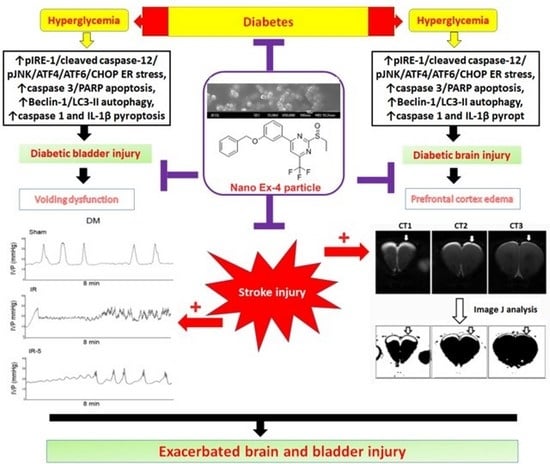
Long-Lasting Exendin-4-Loaded PLGA Nanoparticles Ameliorate Cerebral Ischemia/Reperfusion Damage in Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Chemicals
2.3. Experimental Design and Preparation of Ex-4-Loaded PLGA Nanoparticles
2.4. Nanoparticles Characterization
2.5. In Vivo Drug Release Study
2.6. Animals and Grouping
2.7. Global Cerebral Ischemia
2.8. Cerebral Edema Measurement by T2-Weighted Magnetic Resonance Imaging (MRI)
2.9. Measurement of Specific CSF ROS Activity
2.10. Cystometric Parameters
2.11. Immunohistochemistry
2.12. Western Blotting
2.13. Histologic Staining
2.14. Statistical Analysis
3. Results
3.1. PEx-4 Exerts a More Long-Lasting Hypoglycemic Effect than Ex-4
3.2. PEx-4 Is More Efficient than Ex-4 on Reduction IR-Enhanced RH2O2 and RHOCl Activity in CSF
3.3. PEx-4 Is More Efficient than Ex-4 on Decreasing IR-Induced Brain Edema
3.4. PEx-4 Was More Efficient than Ex-4 on Reducing IR-Enhanced Neuronal Shrinkage and ER Stress in Brains
3.5. PEx-4 Was More Efficient than Ex-4 on Decreasing ER Stress-, Apoptosis-, Pyroptosis- and Autophagy-Related Protein Expression in Rat Brain with DM or IR Injury
3.6. PEx-4 Was More Efficient than Ex-4 in Depressing Pyroptosis, Autophagy and Apoptosis Immunofluorescent Staining in DM or IR Brains
3.7. Ex-4 and PEx-4 Produced Less Protective Effect on Cystometry in Eight Groups of Rats
3.8. IR, Ex-4 or PEx-4 Had No Significant Effect on Bladder Fibrosis in Eight Groups of Rats
3.9. PEx-4 Did Not Produce Better Protection than Ex-4 on Stress-Associated Proteins in Rat Bladders with DM or IR Injury
3.10. PEx-4 Was More Efficient than Ex-4 in Reduction of Apoptosis, Not Pyroptosis and Autophagy in Bladders with DM and IR Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genuth, S.; Alberti, K.G.; Bennett, P.; Buse, J.; Defronzo, R.; Kahn, R.; Kitzmiller, J.; Knowler, W.C.; Lebovitz, H.; Lernmark, A.; et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003, 26, 3160–3167. [Google Scholar] [PubMed] [Green Version]
- Daneshgari, F.; Liu, G.; Birder, L.; Hanna-Mitchell, A.T.; Chacko, S. Diabetic bladder dysfunction: Current translational knowledge. J. Urol. 2009, 182 (Suppl. S6), S18–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.; Starer, P.; Yang, W.C.; Bhola, A. Analysis of voiding disorders in patients with cerebrovascular accidents. Urology 1990, 35, 265–270. [Google Scholar] [CrossRef]
- Han, K.S.; Heo, S.H.; Lee, S.J.; Jeon, S.H.; Yoo, K.H. Comparison of urodynamics between ischemic and hemorrhagic stroke patients; can we suggest the category of urinary dysfunction in patients with cerebrovascular accident according to type of stroke? Neurourol. Urodyn. 2010, 29, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Tang, Z.; He, C.; Tang, W. Diabetic cystopathy: A review. J. Diabetes 2015, 7, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci. USA 1992, 89, 8641–8645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrén, B. Glucagon-like peptide-1 (GLP-1): A gut hormone of potential interest in the treatment of diabetes. BioEssay 1998, 20, 642–651. [Google Scholar] [CrossRef]
- Eng, J.; Kleinman, W.A.; Singh, L.; Singh, G.; Raufman, J.P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 1992, 267, 7402–7405. [Google Scholar] [CrossRef]
- Göke, R.; Fehmann, H.C.; Linn, T.; Schmidt, H.; Krause, M.; Eng, J.; Göke, B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J. Biol. Chem. 1993, 268, 19650–19655. [Google Scholar] [CrossRef]
- Edwards, C.M.B.; Stanley, S.A.; Davis, R.; Brynes, A.E.; Frost, G.S.; Seal, L.J.; Ghatei, M.A.; Bloom, S.R. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E155–E161. [Google Scholar] [CrossRef]
- Nielsen, L.L.; Young, A.A.; Parkes, D.G. Pharmacology of exenatide (synthetic exendin-4): A potential therapeutic for improved glycemic control of type 2 diabetes. Regul. Pept. 2004, 117, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Iltz, J.L.; Baker, D.E.; Setter, S.M.; Campbell, R.K. Exenatide: An incretin mimetic for the treatment of type 2 diabetes mellitus. Clin. Ther. 2006, 28, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.T.; Fan, S.C.; Lin, S.C.; Kuo, C.C.; Yang, C.H.; Yu, T.Y.; Lee, S.-P.; Cheng, D.-Y.; Li, P.C. Glucagon-like peptide-1 receptor agonist activation ameliorates venous thrombosis-induced arteriovenous fistula failure in chronic kidney disease. Thromb. Haemost. 2014, 112, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, S.; Miyamoto, N.; Yatomi, K.; Tanaka, Y.; Oishi, H.; Arai, H.; Hattori, N.; Urabe, T. Exendin-4, a Glucagon-Like Peptide-1 Receptor Agonist, Provides Neuroprotection in Mice Transient Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2011, 31, 1696–1705. [Google Scholar] [CrossRef] [Green Version]
- Chien, C.T.; Jou, M.J.; Cheng, T.Y.; Yang, C.H.; Yu, T.Y.; Li, P.C. Exendin-4-loaded PLGA microspheres relieve cerebral ischemia/reperfusion injury and neurologic deficits through long-lasting bioactivity-mediated phosphorylated Akt/eNOS signaling in rats. J. Cereb. Blood Flow Metab. 2015, 35, 1790–1803. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Palamoor, M.; Jablonski, M.M. Comparative study on diffusion and evaporation emulsion methods used to load hydrophilic drugs in poly(ortho ester) nanoparticle emulsions. Powder Technol. 2014, 253, 53–62. [Google Scholar] [CrossRef]
- Dâmaso, A.R.; Duarte, F.O.; Sene-Fiorese, M.; Manzoni, M.S.J.; Rossi, E.A.; Cheik, N.C.; Guerra, R.L.F.; de Oliveira Duarte, A.C.G. Experimental diet models in the investigation of obesity. In Rodent Model as Tools in Ethical Biomedical Research; Andersen, M.L., Tufik, S., Eds.; Springer International Publishing: London, UK, 2015; pp. 503–516. [Google Scholar]
- Huang, K.C.; Yang, C.C.; Lee, K.T.; Chien, C.T. Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water. Kidney Int. 2003, 64, 704–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, B.J.; Liao, C.H.; Mao, S.H.; Chien, C.T. Adipose-derived stem cells and their derived microvesicles ameliorate detrusor overactivity secondary to bilateral partial iliac arterial occlusion-induced bladder ischemia. Int. J. Mol. Sci. 2021, 22, 7000. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jin, H.Y.; Lee, K.A.; Xie, S.H.; Baek, H.S.; Park, T.S. Neuroprotective effect of the glucagon-like peptide-1 receptor agonist, synthetic exendin-4, in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 2011, 164, 1410–1420. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Liu, K.-F.; Silva, M.D.; Meng, X.; Gerriets, T.; Helmer, K.G.; Fenstermacher, J.D.; Sotak, C.H.; Fisher, M. Acute Postischemic Renormalization of the Apparent Diffusion Coefficient of Water is not Associated with Reversal of Astrocytic Swelling and Neuronal Shrinkage in Rats. Am. J. Neuroradiol. 2002, 23, 180–188. [Google Scholar] [PubMed]
- Nakka, V.P.; Gusain, A.; Raghubir, R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox. Res. 2010, 17, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.; Arato, I.; Di Michele, A.; Antognelli, C.; Angelini, L.; Bellucci, C.; Lilli, C.; Boncompagni, S.; Fusella, A.; Bartolini, D.; et al. Effects of Titanium Dioxide Nanoparticles on Porcine Prepubertal Sertoli Cells: An “In Vitro” Study. Front. Endocrinol. 2022, 12, 751915. [Google Scholar] [CrossRef] [PubMed]
- Akel, H.; Csóka, I.; Ambrus, R.; Bocsik, A.; Gróf, I.; Mészáros, M.; Szecskó, A.; Kozma, G.; Veszelka, S.; Deli, M.A.; et al. In Vitro Comparative Study of Solid Lipid and PLGA Nanoparticles Designed to Facilitate Nose-to-Brain Delivery of Insulin. Int. J. Mol. Sci. 2021, 22, 13258. [Google Scholar] [CrossRef]
- Mollaeva, M.R.; Nikolskaya, E.; Beganovskaya, V.; Sokol, M.; Chirkina, M.; Obydennyi, S.; Belykh, D.; Startseva, O.; Mollaev, M.D.; Yabbarov, N. Oxidative Damage Induced by Phototoxic Pheophorbide a 17-Diethylene Glycol Ester Encapsulated in PLGA Nanoparticles. Antioxidants 2021, 10, 1985. [Google Scholar] [CrossRef] [PubMed]
- Mollaeva, M.R.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.D.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, X.; Liang, H.; He, W.; Zhao, X. Mechanism of miR-30b-5p-Loaded PEG-PLGA Nanoparticles for Targeted Treatment of Heart Failure. Front. Pharmacol. 2021, 12, 745429. [Google Scholar] [CrossRef] [PubMed]
- Spindler, L.M.; Feuerhake, A.; Ladel, S.; Günday, C.; Flamm, J.; Günday-Türeli, N.; Türeli, E.; Tovar, G.E.M.; Schindowski, K.; Gruber-Traub, C. Nano-in-micro-particles consisting of PLGA nanoparticles embedded in chitosan microparticles via spray-drying enhances their uptake in the olfactory mucosa. Front. Pharmacol. 2021, 12, 732954. [Google Scholar] [CrossRef] [PubMed]
- Fishman, R.A. Brain edema. N. Engl. J. Med. 1975, 293, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.; Lahiri, D.K.; Chen, D.; Zhou, J.; Shaw, K.T.Y.; Egan, J.M.; Greig, N.H. A Novel Neurotrophic Property of Glucagon-Like Peptide 1: A Promoter of Nerve Growth Factor-Mediated Differentiation in PC12 Cells. J. Pharmacol. Exp. Ther. 2002, 300, 958–966. [Google Scholar] [CrossRef] [Green Version]
- Bertilsson, G.; Patrone, C.; Zachrisson, O.; Andersson, A.; Dannaeus, K.; Heidrich, J.; Kortesmaa, J.; Mercer, A.; Nielsen, E.; Rönnholm, H.; et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Derugin, N.; Manley, G.T.; Verkman, A.S. Reduced brain edema and infarct volume in aquaporin-4 deficient mice after transient focal cerebral ischemia. Neurosci. Lett. 2015, 584, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Andrew, J.; Nathan, P.W. Lesions of the anterior frontal lobes and disturbances of micturition and defaecation. Brain 1964, 87, 233–262. [Google Scholar] [CrossRef] [Green Version]
- Terreberry, R.R.; Neafsey, E.J. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res. 1987, 19, 639–649. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hanai, T.; Yoshioka, N.; Shimizu, N.; Sugiyama, T.; Uemura, H. Medial prefrontal cortex lesions inhibit reflex micturition in anethetized rats. Neurosci. Res. 2006, 54, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Yao, C.A.; Yang, J.C.; Chien, C.T. Sialic acid rescues repurified lipopolysaccharide-induced acute renal failure via inhibiting TLR4/PKC/gp91-mediated endoplasmic reticulum stress, apoptosis, autophagy, and pyroptosis signaling. Toxicol. Sci. 2014, 141, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Hendershot, L.M. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 2004, 28, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toniolo, A.; Warden, E.A.; Nassi, A.; Cignarella, A.; Bolego, C. Regulation of SIRT1 in vascular smooth muscle cells from streptozotocin-diabetic rats. PLoS ONE 2013, 8, e65666. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Hong, S.-W.; Park, S.E.; Rhee, E.-J.; Park, C.-Y.; Oh, K.-W.; Park, S.-W.; Lee, W.-Y. Exendin-4 attenuates endoplasmic reticulum stress through a SIRT1-dependent mechanism. Cell Stress Chaperone 2014, 19, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Kaneto, H.; Laybutt, D.R.; Duvivier-Kali, V.F.; Trivedi, N.; Suzuma, K.; King, G.L.; Weir, G.C.; Bonner-Weir, S. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: Possible contribution to impaired incretin effects in diabetes. Diabetes 2007, 56, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Tsunekawa, S.; Yamamoto, N.; Tsukamoto, K.; Itoh, Y.; Kaneko, Y.; Kimura, T.; Ariyoshi, Y.; Miura, Y.; Oiso, Y.; Niki, I. Protection of pancreatic beta-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. J. Endocrinol. 2007, 193, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Zhang, J.; Lei, X.; Xu, G.T.; Ye, W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Younce, C.W.; Burmeister, M.A.; Ayala, J.E. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. Am. J. Physiol. Cell Physiol. 2012, 304, C508–C518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darsalia, V.; Hua, S.; Larsson, M.; Mallard, C.; Nathanson, D.; Nyström, T.; Sjöholm, Å.; Johansson, M.; Patrone, C. Exendin-4 Reduces Ischemic Brain Injury in Normal and Aged Type 2 Diabetic Mice and Promotes Microglial M2 Polarization. PLoS ONE 2014, 9, e103114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastin, A.J.; Akerstrom, V.; Pan, W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood–brain barrier. J. Mol. Neurosci. 2002, 18, 7–14. [Google Scholar] [CrossRef]
- Gelber, D.A.; Good, D.C.; Laven, L.J.; Verhulst, S.J. Causes of urinary incontinence after acute hemispheric stroke. Stroke 1993, 24, 378–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yotsuyanagi, S.; Narimoto, K.; Namiki, M. Mild brain ischemia produces bladder hyperactivity without brain damage in rats. Urol. Int. 2006, 77, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Sellers, D.J.; McKay, N.G.; Chapple, C.R.; Chess-Williams, R. Muscarinic receptor function, density and G-protein coupling in the overactive diabetic rat bladder. Auton. Autacoid Pharmacol. 2006, 26, 303–309. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, C.-H.; Chung, S.-D.; Cheng, Y.-H.; Yang, C.-P.; Chien, C.-T. Long-Lasting Exendin-4-Loaded PLGA Nanoparticles Ameliorate Cerebral Ischemia/Reperfusion Damage in Diabetic Rats. J. Pers. Med. 2022, 12, 390. https://doi.org/10.3390/jpm12030390
Chung C-H, Chung S-D, Cheng Y-H, Yang C-P, Chien C-T. Long-Lasting Exendin-4-Loaded PLGA Nanoparticles Ameliorate Cerebral Ischemia/Reperfusion Damage in Diabetic Rats. Journal of Personalized Medicine. 2022; 12(3):390. https://doi.org/10.3390/jpm12030390
Chicago/Turabian StyleChung, Cheng-Hsun, Shiu-Dong Chung, Yu-Hsuan Cheng, Chun-Pai Yang, and Chiang-Ting Chien. 2022. "Long-Lasting Exendin-4-Loaded PLGA Nanoparticles Ameliorate Cerebral Ischemia/Reperfusion Damage in Diabetic Rats" Journal of Personalized Medicine 12, no. 3: 390. https://doi.org/10.3390/jpm12030390
APA StyleChung, C. -H., Chung, S. -D., Cheng, Y. -H., Yang, C. -P., & Chien, C. -T. (2022). Long-Lasting Exendin-4-Loaded PLGA Nanoparticles Ameliorate Cerebral Ischemia/Reperfusion Damage in Diabetic Rats. Journal of Personalized Medicine, 12(3), 390. https://doi.org/10.3390/jpm12030390