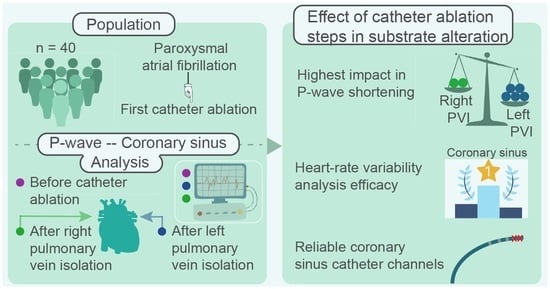
The Dissimilar Impact in Atrial Substrate Modificationof Left and Right Pulmonary Veins Isolation after Catheter Ablation of Paroxysmal Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database
2.2. Preprocessing
2.3. Main Analysis
- Duration: Distance between the onset and offset of each activation.
- Amplitude: Amplitude of positive and negative maximum of each activation were considered as positive (PosAmp) and negative amplitude (NegAmp), respectively. Peak-to-peak amplitude (PPAmp) was the distance between positive and negative maximum points. As P-waves are positive in lead II, only maximum amplitude was calculated for ECG analysis.
- RMS: Let be a time-series, so that . RMS value is the quadratic mean of the function that defines the time-series. In our case, this function is defined by either the P-wave or LAW waveform.
- Area: Area is calculated as the integration of the signal over the time interval. Trapezoidal method allows this integration, by splitting each signal into smaller and easier to calculate trapezoids. Final area is defined by the cumulative sum of these trapezoids. As LAWs contain both positive (PosAr) and negative (NegAr) parts, this method was separately applied to each one of them.
- NODI: Deflections and inflections were calculated from the points that cross two auxiliary baselines, at of the signal amplitude. This metric was only calculated for LAWs, as P-waves do not show multiple major deflections and inflections.
- Slope rate: The rhythm of increasing or decreasing slope was calculated at sample points equal to of the activation duration, with . Slope rate at the maximum point was also computed. The equation calculating these slope rates was the following:where is the amplitude at the of the activation duration, is the amplitude at the onset of the activation, is the sample point at the of the activation duration and is the sample point corresponding to the onset of the activation.
- MV: A reference signal was firstly created by the 20 most similar activations of the channel under analysis and then correlated with each and every activation, using an adaptive signed correlation index (ASCI) with tolerance [67]. MV was then defined as the percentage of signals that correlated <95% with the reference signal.
- Dispersion: Traditionally, for the calculation of dispersion, more than one ECG lead is employed and the difference between maximum and minimum activation duration across channels is computed. Alternatively, in our case, lead II was just extracted and P-wave dispersion analysis was performed in this channel because atrial activity presents the highest amplitude. Dispersion was then defined as the difference between the 25th and 75th percentiles of the atrial activations duration of each recording. This way, the effect of signal delineation accuracy is minimized and an extremely long or short activation caused by various factors will not affect significantly the results [68]. Dispersion of EGM recordings was calculated in the same way.
- Time-domain HRV features: HRV analysis is normally performed on R-R intervals, thus describing ventricular response. As in the present work we are focused on atrial analysis, we modified the techniques by substituting R-R peaks by P-wave to P-wave for ECG and LAW to LAW for EGM recordings. As these features describe the atrial response, thus neglecting the effect of the atrioventricular node and other cardiac structures, they will be referred in the remainder of this document as atrial rate variability (ARV) features. Standard deviation of normal-to-normal beat interval (SDNN), variance of normal to normal beat interval (VARNN) and RMS of successive interbeat differences (RMSSD) were calculated for each recording.
Heart Rate Adjustment
2.4. Statistical Analysis
3. Results
3.1. Analysis of CS Features between Channels
3.2. Analysis of Features from P-Waves and LAWs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstro, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 374–498. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannidis, P.; Zografos, T.; Christoforatou, E.; Kouvelas, K.; Tsoumeleas, A.; Vassilopoulos, C. The Electrophysiology of Atrial Fibrillation: From Basic Mechanisms to Catheter Ablation. Cardiol. Res. Pract. 2021, 2021, 4109269. [Google Scholar] [CrossRef]
- Lau, D.H.; Linz, D.; Schotten, U.; Mahajan, R.; Sanders, P.; Kalman, J.M. Pathophysiology of Paroxysmal and Persistent Atrial Fibrillation: Rotors, Foci and Fibrosis. Heart Lung Circ. 2017, 26, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Mouws, E.M.J.P.; Lanters, E.A.H.; Teuwen, C.P.; van der Does, L.J.M.E.; Kik, C.; Knops, P.; Bekkers, J.A.; Bogers, A.J.J.C.; de Groot, N.M.S. Epicardial Breakthrough Waves During Sinus Rhythm: Depiction of the Arrhythmogenic Substrate? Circ. Arrhythmia Electrophysiol. 2017, 10, e005145. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, A.; Nothstein, M.; Chen, J.; Lehrmann, H.; Dössel, O.; Allgeier, J.; Trenk, D.; Neumann, F.J.; Loewe, A.; Müller-Edenborn, B.; et al. Specific Electrogram Characteristics Identify the Extra-Pulmonary Vein Arrhythmogenic Sources of Persistent Atrial Fibrillation—Characterization of the Arrhythmogenic Electrogram Patterns During Atrial Fibrillation and Sinus Rhythm. Sci. Rep. 2020, 10, 9147. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; Knops, P.; van der Does, L.; Kik, C.; Taverne, Y.; Roos-Serote, M.C.; Heida, A.; Oei, F.; Bogers, A.; de Groot, N. Simultaneous Endo-Epicardial Mapping of the Human Right Atrium: Unraveling Atrial Excitation. J. Am. Heart Assoc. 2020, 9, e017069. [Google Scholar] [CrossRef]
- Shah, D.; Haïssaguerre, M.; Jaïs, P. Catheter Ablation of Pulmonary Vein Foci for Atrial Fibrillation. Thorac. Cardiovasc. Surg. 1999, 47, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.; Knight, B.P.; Tada, H. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002, 11, 83. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Tarantino, N.; Trivedi, C.; Mohanty, S.; Anannab, A.; Salwan, A.S.; Gianni, C.; Bassiouny, M.; Al-Ahmad, A.; Romero, J.; et al. Non-pulmonary vein triggers in nonparoxysmal atrial fibrillation: Implications of pathophysiology for catheter ablation. J. Cardiovasc. Electrophysiol. 2020, 31, 2154–2167. [Google Scholar] [CrossRef]
- Lin, W.S.; Tai, C.T.; Hsieh, M.H. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation 2003, 12, 53. [Google Scholar] [CrossRef]
- Sánchez-Quintana, D.; López-Mínguez, J.R.; Pizarro, G.; Murillo, M.; Cabrera, J.A. Triggers and anatomical substrates in the genesis and perpetuation of atrial fibrillation. Curr. Cardiol. Rev. 2012, 8, 310–326. [Google Scholar] [CrossRef] [Green Version]
- Nademanee, K.; McKenzie, J.; Kosar, E.; Schwab, M.; Sunsaneewitayakul, B.; Vasavakul, T.; Khunnawat, C.; Ngarmukos, T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004, 43, 2044–2053. [Google Scholar] [CrossRef] [Green Version]
- Vraka, A.; Hornero, F.; Bertomeu-González, V.; Osca, J.; Alcaraz, R.; Rieta, J.J. Short-Time Estimation of Fractionation in Atrial Fibrillation with Coarse-Grained Correlation Dimension for Mapping the Atrial Substrate. Entropy 2020, 22, 232. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.L.; Tai, C.T.; Lin, Y.J.; Wongcharoen, W.; Lo, L.W.; Tuan, T.C.; Udyavar, A.R.; Chang, S.H.; Tsao, H.M.; Hsieh, M.H.; et al. Biatrial substrate properties in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007, 18, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Garg, L.; Pothineni, N.V.K.; Daw, J.M.; Hyman, M.C.; Arkles, J.; Tschabrunn, C.M.; Santangeli, P.; Marchlinski, F.E. Impact of Left Atrial Bipolar Electrogram Voltage on First Pass Pulmonary Vein Isolation During Radiofrequency Catheter Ablation. Front. Physiol. 2020, 11, 594654. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Lo, L.W.; Lin, Y.J.; Chang, S.L.; Hu, Y.F.; Hung, Y.; Chung, F.P.; Liao, J.N.; Tuan, T.C.; Chao, T.F.; et al. Ten-year ablation outcomes of patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Heart Rhythm 2019, 16, 1327–1333. [Google Scholar] [CrossRef]
- Inamura, Y.; Nitta, J.; Inaba, O.; Sato, A.; Takamiya, T.; Murata, K.; Ikenouchi, T.; Kono, T.; Matsumura, Y.; Takahashi, Y.; et al. Presence of non-pulmonary vein foci in patients with atrial fibrillation undergoing standard ablation of pulmonary vein isolation: Clinical characteristics and long-term ablation outcome. Int. J. Cardiol. Heart Vasc. 2021, 32, 100717. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Foster, J.R.; Gettes, L.S. Atrial excitability and conduction in patients with interatrial conduction defects. Am. J. Cardiol. 1982, 50, 1331–1337. [Google Scholar] [CrossRef]
- Blanche, C.; Tran, N.; Rigamonti, F.; Burri, H.; Zimmermann, M. Value of P-wave signal averaging to predict atrial fibrillation recurrences after pulmonary vein isolation. EP Eur. 2013, 15, 198–204. [Google Scholar] [CrossRef]
- Chen, Q.; Mohanty, S.; Trivedi, C.; Gianni, C.; Della Rocca, D.G.; Canpolat, U.; Burkhardt, J.D.; Sanchez, J.E.; Hranitzky, P.; Gallinghouse, G.J.; et al. Association between prolonged P wave duration and left atrial scarring in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2019, 30, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Yonas, E.; Vania, R. Prolonged P-wave duration in sinus rhythm pre-ablation is associated with atrial fibrillation recurrence after pulmonary vein isolation—A systematic review and meta-analysis. Ann. Noninvasive Electrocardiol. 2019, 24, e12653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auricchio, A.; Özkartal, T.; Salghetti, F.; Neumann, L.; Pezzuto, S.; Gharaviri, A.; Demarchi, A.; Caputo, M.L.; Regoli, F.; De Asmundis, C.; et al. Short P-Wave Duration is a Marker of Higher Rate of Atrial Fibrillation Recurrences after Pulmonary Vein Isolation: New Insights into the Pathophysiological Mechanisms Through Computer Simulations. J. Am. Heart Assoc. 2021, 10, e018572. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Xu, M.; Yang, L.; Zhang, C.; Liu, H.; Shao, X. Investigating the association between P wave duration and atrial fibrillation recurrence after radiofrequency ablation in early persistent atrial fibrillation patients. Int. J. Cardiol. 2021, 351, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Van Beeumen, K.; Houben, R.; Tavernier, R.; Ketels, S.; Duytschaever, M. Changes in P-wave area and P-wave duration after circumferential pulmonary vein isolation. EP Eur. 2010, 12, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Maan, A.; Mansour, M.; Ruskin, J.N.; Heist, E.K. Impact of catheter ablation on P-wave parameters on 12-lead electrocardiogram in patients with atrial fibrillation. J. Electrocardiol. 2014, 47, 725–733. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, J.; Ma, Y.; Tang, A. Novel P Wave Indices to Predict Atrial Fibrillation Recurrence After Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. Med. Sci. Monit. 2016, 22, 2616–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vraka, A.; Bertomeu-González, V.; Hornero, F.; Quesada, A.; Alcaraz, R.; Rieta, J.J. Splitting the P-Wave: Improved Evaluation of Left Atrial Substrate Modification after Pulmonary Vein Isolation of Paroxysmal Atrial Fibrillation. Sensors 2022, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, R.; Martínez, A.; Rieta, J.J. The P Wave Time-Frequency Variability Reflects Atrial Conduction Defects before Paroxysmal Atrial Fibrillation. Ann. Noninvasive Electrocardiol. 2015, 20, 433–445. [Google Scholar] [CrossRef]
- Murase, Y.; Imai, H.; Ogawa, Y.; Kano, N.; Mamiya, K.; Ikeda, T.; Okabe, K.; Arai, K.; Yamazoe, S.; Torii, J.; et al. Usefulness of P-wave duration in patients with sick sinus syndrome as a predictor of atrial fibrillation. J. Arrhythmia 2021, 37, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Dong, J.Z.; Sang, C.H.; Tang, R.B.; Ma, C.S. Prolonged PR interval and risk of recurrence of atrial fibrillation after catheter ablation. Int. Heart J. 2014, 55, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Salah, A.; Zhou, S.; Liu, Q.; Yan, H. P wave indices to predict atrial fibrillation recurrences post pulmonary vein isolation. Arq. Bras. Cardiol. 2013, 101, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, R.; Martínez, A.; Rieta, J.J. Role of the P-wave high frequency energy and duration as noninvasive cardiovascular predictors of paroxysmal atrial fibrillation. Comput. Methods Programs Biomed. 2015, 119, 110–119. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiologys. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Perkiömäki, J.; Ukkola, O.; Kiviniemi, A.; Tulppo, M.; Ylitalo, A.; Kesäniemi, Y.A.; Huikuri, H. Heart rate variability findings as a predictor of atrial fibrillation in middle-aged population. J. Cardiovasc. Electrophysiol. 2014, 25, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Chahal, H.; Greenland, P.; Guallar, E.; Lima, J.A.C.; Soliman, E.Z.; Alonso, A.; Heckbert, S.R.; Nazarian, S. Resting Heart Rate, Short-Term Heart Rate Variability and Incident Atrial Fibrillation (from the Multi-Ethnic Study of Atherosclerosis (MESA)). Am. J. Cardiol. 2019, 124, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Chiou, C.W.; Wen, Z.C.; Wu, C.H.; Tai, C.T.; Tsai, C.F.; Ding, Y.A.; Chang, M.S.; Chen, S.A. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation 1999, 100, 2237–2243. [Google Scholar] [CrossRef]
- Chen, P.S.; Chen, L.S.; Fishbein, M.C.; Lin, S.F.; Nattel, S. Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy. Circ. Res. 2014, 114, 1500–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Wang, W.; Cheng, Y.; Wang, X.; Sun, J. The predictive value of heart rate variability indices tested in early period after radiofrequency catheter ablation for the recurrence of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 1350–1355. [Google Scholar] [CrossRef]
- Maille, B.; Das, M.; Hussein, A.; Shaw, M.; Chaturvedi, V.; Williams, E.; Morgan, M.; Ronayne, C.; Snowdon, R.L.; Gupta, D. Reverse electrical and structural remodeling of the left atrium occurs early after pulmonary vein isolation for persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2020, 58, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Daoud, E.G.; Niebauer, M.; Bakr, O.; Jentzer, J.; Man, K.C.; Williamson, B.D.; Hummel, J.D.; Strickberger, S.A.; Morady, F. Placement of electrode catheters into the coronary sinus during electrophysiology procedures using a femoral vein approach. Am. J. Cardiol. 1994, 74, 194–195. [Google Scholar] [CrossRef] [Green Version]
- Antz, M.; Otomo, K.; Arruda, M.; Scherlag, B.J.; Pitha, J.; Tondo, C.; Lazzara, R.; Jackman, W.M. Electrical conduction between the right atrium and the left atrium via the musculature of the coronary sinus. Circulation 1998, 98, 1790–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangeli, P.; Marchlinski, F.E. Techniques for the provocation, localization, and ablation of non–pulmonary vein triggers for atrial fibrillation. Heart Rhythm 2017, 14, 1087–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.; Perveen, S.; Mehmood, A.; Rani, G.F.; Molon, G. Coronary Sinus Ablation Is a Key Player Substrate in Recurrence of Persistent Atrial Fibrillation. Cardiology 2019, 143, 107–113. [Google Scholar] [CrossRef]
- Razeghian-Jahromi, I.; Natale, A.; Nikoo, M.H. Coronary sinus diverticulum: Importance, function, and treatment. Pacing Clin. Electrophysiol. PACE 2020, 43, 1582–1587. [Google Scholar] [CrossRef]
- Boles, U.; Gul, E.E.; Enriquez, A.; Starr, N.; Haseeb, S.; Abdollah, H.; Simpson, C.; Baranchuk, A.; Redfearn, D.; Michael, K.; et al. Coronary sinus electrograms may predict new-onset atrial fibrillation after typical atrial flutter radiofrequency ablation. J. Atr. Fibrillation 2018, 11, 1809. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Zipes, D.P.; Morita, S.T.; Wu, J. The role of coronary sinus musculature in the induction of atrial fibrillation. Heart Rhythm 2012, 9, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Kato, R.; Ikeda, Y.; Goto, K.; Asano, S.; Mori, H.; Iwanaga, S.; Muramatsu, T.; Matsumoto, K. Differential Atrial Pacing to Detect Reconnection Gaps After Pulmonary Vein Isolation in Atrial Fibrillation. Int. Heart J. 2020, 61, 503–509. [Google Scholar] [CrossRef]
- Mahmud, E.; Raisinghani, A.; Keramati, S.; Auger, W.; Blanchard, D.G.; DeMaria, A.N. Dilation of the coronary sinus on echocardiogram: Prevalence and significance in patients with chronic pulmonary hypertension. JASE 2001, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.J.M.; Pietersen, H.G.; Geskes, G.; Wagenmakers, A.J.M.; Soeters, P.B.; Durieux, M. Coronary Sinus Catheter Placement. Clin. Investig. Cardiol. 2003, 124, 1259–1265. [Google Scholar] [CrossRef]
- Saremi, F.; Thonar, B.; Sarlaty, T.; Shmayevich, I.; Malik, S.; Smith, C.W.; Krishnan, S.; Sánchez-Quintana, D.; Narula, N. Posterior interatrial muscular connection between the coronary sinus and left atrium: Anatomic and functional study of the coronary sinus with multidetector CT. Radiology 2011, 260, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Heintze, J.; Hansky, B.; Güldner, H.; Buschler, H.; Horstkotte, D. Implantation: Tips and tricks–the cardiologist’s view. Eur. Heart J. Suppl. 2004, 6, D47–D52. [Google Scholar] [CrossRef]
- Pai, R.G.; Varadarajan, P.; Tanimoto, M. Effect of atrial fibrillation on the dynamics of mitral annular area. J. Heart Valve Dis. 2003, 12, 31–37. [Google Scholar] [PubMed]
- El-Maasarany, S.; Ferrett, C.G.; Firth, A.; Sheppard, M.; Henein, M.Y. The coronary sinus conduit function: Anatomical study (relationship to adjacent structures). EP Eur. 2005, 7, 475–481. [Google Scholar] [CrossRef]
- Meek, S.; Morris, F. ABC of clinical electrocardiography: Introduction. II—basic terminology. BMJ 2002, 324, 470–473. [Google Scholar] [CrossRef]
- García, M.; Martínez-Iniesta, M.; Ródenas, J.; Rieta, J.J.; Alcaraz, R. A novel wavelet-based filtering strategy to remove powerline interference from electrocardiograms with atrial fibrillation. Physiol. Meas. 2018, 39, 115006. [Google Scholar] [CrossRef] [PubMed]
- Sörnmo, L.; Laguna, P. Electrocardiogram (ECG) Signal Processing. In Wiley Encyclopedia of Biomedical Engineering; John Wiley and Sons: Hoboken, NJ, USA, 2006; Volume 2, pp. 1298–1313. [Google Scholar] [CrossRef]
- Martínez-Iniesta, M.; Ródenas, J.; Rieta, J.J.; Alcaraz, R. The stationary wavelet transform as an efficient reductor of powerline interference for atrial bipolar electrograms in cardiac electrophysiology. Physiol. Meas. 2019, 40, 075003. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, R.; Rieta, J.J. Adaptive singular value cancelation of ventricular activity in single-lead atrial fibrillation electrocardiograms. Physiol. Meas. 2008, 29, 1351–1369. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Alcaraz, R.; Rieta, J.J. Detection and removal of ventricular ectopic beats in atrial fibrillation recordings via principal component analysis. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 4693–4696. [Google Scholar] [CrossRef]
- Choi, A.; Shin, H. Quantitative Analysis of the Effect of an Ectopic Beat on the Heart Rate Variability in the Resting Condition. Front. Physiol. 2018, 9, 922. [Google Scholar] [CrossRef]
- Martinez, A.; Alcaraz, R.; Rieta, J.J. A new method for automatic delineation of ECG fiducial points based on the Phasor Transform. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4586–4589. [Google Scholar] [CrossRef]
- González, F.; Alcaraz, R.; Rieta, J.J. Electrocardiographic P-wave delineation based on adaptive slope Gaussian detection. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Osorio, D.; Alcaraz, R.; Rieta, J.J. A fractionation-based local activation wave detector for atrial electrograms of atrial fibrillation. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Vraka, A.; Bertomeu-González, V.; Osca, J.; Ravelli, F.; Alcaraz, R.; Rieta, J.J. Study on How Catheter Ablation Affects Atrial Structures in Patients with Paroxysmal Atrial Fibrillation: The Case of the Coronary Sinus. In Proceedings of the 2020 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 29–30 October 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Alcaraz, R.; Hornero, F.; Martínez, A.; Rieta, J.J. Short-time regularity assessment of fibrillatory waves from the surface ECG in atrial fibrillation. Physiol. Meas. 2012, 33, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, J.; Zawadzki, G.; Radziejewska, J.; Wolff, P.S.; Sławuta, A.; Gajek, J. The P wave dispersion—One pixel, one millisecond. Rev. Cardiovasc. Med. 2021, 22, 1633. [Google Scholar] [CrossRef] [PubMed]
- Toman, O.; Hnatkova, K.; Smetana, P.; Huster, K.M.; Šišáková, M.; Barthel, P.; Novotný, T.; Schmidt, G.; Malik, M. Physiologic heart rate dependency of the PQ interval and its sex differences. Sci. Rep. 2020, 10, 2551. [Google Scholar] [CrossRef] [PubMed]
- Mandelbrot, B.B. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Siam Rev. 1961, 3, 80. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Oral, H.; Ozaydin, M.; Chugh, A.; Scharf, C.; Tada, H.; Hall, B.; Cheung, P.; Pelosi, F.; Knight, B.P.; Morady, F. Role of the coronary sinus in maintenance of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2003, 14, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Di Marco, L.Y.; Raine, D.; Bourke, J.P.; Langley, P. Characteristics of atrial fibrillation cycle length predict restoration of sinus rhythm by catheter ablation. Heart Rhythm 2013, 10, 1303–1310. [Google Scholar] [CrossRef]
- Misek, J.; Belyaev, I.; Jakusova, V.; Tonhajzerova, I.; Barabas, J.; Jakus, J. Heart rate variability affected by radiofrequency electromagnetic field in adolescent students. Bioelectromagnetics 2018, 39, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Misek, J.; Veternik, M.; Tonhajzerova, I.; Jakusova, V.; Janousek, L.; Jakus, J. Radiofrequency Electromagnetic Field Affects Heart Rate Variability in Rabbits. Physiol. Res. 2020, 69, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Ortigosa, N.; Ayala, G.; Cano, Ó. Variation of P-wave indices in paroxysmal atrial fibrillation patients before and after catheter ablation. Biomed. Signal Process. Control 2021, 66, 102500. [Google Scholar] [CrossRef]
- McCann, A.; Vesin, J.M.; Pruvot, E.; Roten, L.; Sticherling, C.; Luca, A. ECG-Based Indices to Characterize Persistent Atrial Fibrillation Before and During Stepwise Catheter Ablation. Front. Physiol. 2021, 12, 654053. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Pradella, M.; Reichlin, T.; Mühl, A.; Bossard, M.; Stieltjes, B.; Conen, D.; Bremerich, J.; Osswald, S.; Kühne, M.; et al. Left atrial anatomy, atrial fibrillation burden, and P-wave duration—Relationships and predictors for single-procedure success after pulmonary vein isolation. EP Eur. 2018, 20, 271–278. [Google Scholar] [CrossRef]





| KW | MWU | |||||||
|---|---|---|---|---|---|---|---|---|
| D-M | D-MP | D-P | MD-M | MD-MP | M-P | MP-P | ||
| Duration | ||||||||
| PosAmp | <* | <* | <* | * | ||||
| NegAmp | * | * | * | |||||
| PPAmp | * | <* | * | |||||
| RMS | * | * | * | |||||
| PosAr | * | * | * | |||||
| NegAr | * | * | * | |||||
| Deflections | ||||||||
| Inflections | ||||||||
| Features | Distal | Mid-Distal | Medial | Mid-Proximal | Proximal |
|---|---|---|---|---|---|
| Duration | |||||
| PosAmp | <* | * | * | ||
| NegAmp | * | * | |||
| PPAmp | * | * | |||
| RMS | * | * | |||
| PosAr | * | * | |||
| NegAr | * | ||||
| Deflections | |||||
| Inflections |
| B | L | R | |
|---|---|---|---|
| Median (iqr) | |||
| KW | |||
| Median | KW | MWU | |||||
|---|---|---|---|---|---|---|---|
| Features | B | L | R | B-L | B-R | L-R | |
| Duration [ms] | * | * | * | ||||
| PosAmp [mV] | |||||||
| PPAmp [mV] | |||||||
| RMS [mV] | |||||||
| PosAr [mV×ms] | |||||||
| [mV/ms] | ) | ||||||
| [mV/ms] | |||||||
| [mV/ms] | |||||||
| [mV/ms] | |||||||
| MV | |||||||
| Dispersion [ms] | |||||||
| SDNN | |||||||
| VARNN | |||||||
| RMSSD | |||||||
| Median | KW | MWU | |||||
|---|---|---|---|---|---|---|---|
| Features | B | L | R | B-L | B-R | L-R | |
| Duration [ms] | |||||||
| PosAmp [mV] | |||||||
| NegAmp [mV] | |||||||
| PPAmp [mV] | |||||||
| RMS [mV] | |||||||
| PosAr [mV×ms] | |||||||
| NegAr [mV×ms] | |||||||
| Deflections | |||||||
| Inflections | |||||||
| [mV/ms] | |||||||
| [mV/ms] | |||||||
| [mV/ms] | |||||||
| [mV/ms] | |||||||
| MV | |||||||
| Dispersion [ms] | |||||||
| SDNN | |||||||
| VARNN | |||||||
| RMSSD | |||||||
| B-L [%] | L-R [%] | B-R [%] | MWU (BL-LR) | |||||
|---|---|---|---|---|---|---|---|---|
| Features | P-Waves | LAWs | P-Waves | LAWs | P-Waves | LAWs | P-Waves | LAWs |
| Duration | < | |||||||
| PosAmp | ||||||||
| PPAmp | ||||||||
| RMS | ||||||||
| PosAr | ||||||||
| MV | * | |||||||
| Dispersion | ||||||||
| SDNN | * | |||||||
| VARNN | * | |||||||
| RMSSD | * | * | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vraka, A.; Bertomeu-González, V.; Fácila, L.; Moreno-Arribas, J.; Alcaraz, R.; Rieta, J.J. The Dissimilar Impact in Atrial Substrate Modificationof Left and Right Pulmonary Veins Isolation after Catheter Ablation of Paroxysmal Atrial Fibrillation. J. Pers. Med. 2022, 12, 462. https://doi.org/10.3390/jpm12030462
Vraka A, Bertomeu-González V, Fácila L, Moreno-Arribas J, Alcaraz R, Rieta JJ. The Dissimilar Impact in Atrial Substrate Modificationof Left and Right Pulmonary Veins Isolation after Catheter Ablation of Paroxysmal Atrial Fibrillation. Journal of Personalized Medicine. 2022; 12(3):462. https://doi.org/10.3390/jpm12030462
Chicago/Turabian StyleVraka, Aikaterini, Vicente Bertomeu-González, Lorenzo Fácila, José Moreno-Arribas, Raúl Alcaraz, and José J. Rieta. 2022. "The Dissimilar Impact in Atrial Substrate Modificationof Left and Right Pulmonary Veins Isolation after Catheter Ablation of Paroxysmal Atrial Fibrillation" Journal of Personalized Medicine 12, no. 3: 462. https://doi.org/10.3390/jpm12030462
APA StyleVraka, A., Bertomeu-González, V., Fácila, L., Moreno-Arribas, J., Alcaraz, R., & Rieta, J. J. (2022). The Dissimilar Impact in Atrial Substrate Modificationof Left and Right Pulmonary Veins Isolation after Catheter Ablation of Paroxysmal Atrial Fibrillation. Journal of Personalized Medicine, 12(3), 462. https://doi.org/10.3390/jpm12030462