Prostate-Specific Membrane Antigen (PSMA) Expression in Tumor-Associated Neovasculature Is an Independent Prognostic Marker in Patients with Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunohistochemistry
2.3. Data Analysis and Statistics
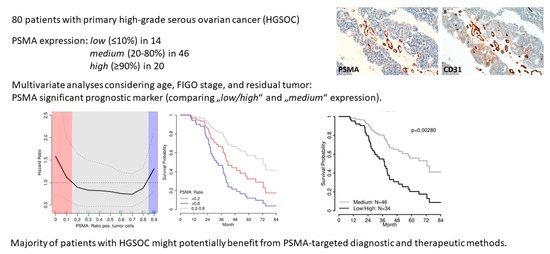
3. Results
3.1. Study Population
3.2. Immunohistochemistry
3.3. Survival Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Haffner, M.C.; Laimer, J.; Chaux, A.; Schäfer, G.; Obrist, P.; Brunner, A.; Kronberger, I.E.; Laimer, K.; Gurel, B.; Koller, J.B.; et al. High expression of prostate-specific membrane antigen in the tumor-associated neo-vasculature is associated with worse prognosis in squamous cell carcinoma of the oral cavity. Mod. Pathol. 2012, 25, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; di Tommaso, L.; Kirienko, M.; Piombo, C.; Erreni, M.; Lania, A.G.; Erba, P.A.; Antunovic, L.; Chiti, A. PSMA expression level predicts differentiated thyroid cancer aggressiveness and patient outcome. EJNMMI Res. 2019, 9, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernicke, A.G.; Varma, S.; Greenwood, E.A.; Christos, P.J.; Chao, K.S.; Liu, H.; Bander, N.H.; Shin, S.J. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS 2014, 122, 482–489. [Google Scholar] [CrossRef]
- Jiao, D.; Li, Y.; Yang, F.; Han, D.; Wu, J.; Shi, S.; Tian, F.; Guo, Z.; Xi, W.; Li, G.; et al. Expression of Prostate-Specific Membrane Antigen in Tumor-Associated Vasculature Predicts Poor Prognosis in Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 2019, 10, e00041. [Google Scholar] [CrossRef]
- Spatz, S.; Tolkach, Y.; Jung, K.; Stephan, C.; Busch, J.; Ralla, B.; Rabien, A.; Feldmann, G.; Brossart, P.; Bundschuh, R.A.; et al. Comprehensive Evaluation of Prostate Specific Membrane Antigen Expression in the Vasculature of Renal Tumors: Implications for Imaging Studies and Prognostic Role. J. Urol. 2018, 199, 370–377. [Google Scholar] [CrossRef]
- Traub-Weidinger, T.; Poetsch, N.; Woehrer, A.; Klebermass, E.M.; Bachnik, T.; Preusser, M.; Mischkulnig, M.; Kiesel, B.; Widhalm, G.; Mitterhauser, M.; et al. PSMA Expression in 122 Treatment Naive Glioma Patients Related to Tumor Metabolism in 11C-Methionine PET and Survival. J. Pers. Med. 2021, 11, 624. [Google Scholar] [CrossRef]
- Israeli, R.S.; Powell, C.T.; Fair, W.R.; Heston, W.D. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993, 53, 227–230. [Google Scholar]
- Rajasekaran, A.K.; Anilkumar, G.; Christiansen, J.J. Is prostate-specific membrane antigen a multifunctional protein? Am. J. Physiol. Cell Physiol. 2005, 288, C975–C981. [Google Scholar] [CrossRef] [Green Version]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Uijen, M.J.M.; Derks, Y.H.W.; Merkx, R.I.J.; Schilham, M.G.M.; Roosen, J.; Privé, B.M.; van Lith, S.A.M.; van Herpen, C.M.L.; Gotthardt, M.; Heskamp, S.; et al. PSMA radioligand therapy for solid tumors other than prostate cancer: Background, opportunities, challenges, and first clinical reports. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4350–4368. [Google Scholar] [CrossRef] [PubMed]
- Wernicke, A.G.; Kim, S.; Liu, H.; Bander, N.H.; Pirog, E.C. Prostate-specific Membrane Antigen (PSMA) Expression in the Neovasculature of Gynecologic Malignancies: Implications for PSMA-targeted Therapy. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 271–276. [Google Scholar] [CrossRef]
- Aide, N.; Poulain, L.; Elie, N.; Briand, M.; Giffard, F.; Blanc-Fournier, C.; Joly, F.; Lasnon, C. A PSMA-targeted theranostic approach is unlikely to be efficient in serous ovarian cancers. EJNMMI Res. 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Banerjee, D.; Barnes, P.J.; Berendt, R.C.; Butany, J.; Canil, S.; Clarke, B.A.; El-Zimaity, H.; Garratt, J.; Geldenhuys, L.; et al. Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee for High Complexity Testing/Immunohistochemistry: Guidelines for the preparation, release, and storage of unstained archived diagnostic tissue sections for immunohistochemistry. Am. J. Clin. Pathol. 2014, 142, 629–633. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 28 March 2022).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Zlobec, I.; Terracciano, L.; Jass, J.R.; Lugli, A. Value of staining intensity in the interpretation of immunohistochemistry for tumor markers in colorectal cancer. Virchows Arch. 2007, 451, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.E.; Rojas, C.; Alt, J.; Nováková, Z.; Richardson, S.M.; Rodrick, T.C.; Fuentes, J.L.; Richardson, N.H.; Attalla, J.; Stewart, S.; et al. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis 2016, 19, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, V.; Del Vecchio, V.; Gargano, G.; De Liso, M.; Kardashi, A.; Naglieri, E.; Resta, L.; Cicinelli, E.; Cormio, G. Biological Pathways Involved in Tumor Angiogenesis and Bevacizumab Based Anti-Angiogenic Therapy with Special References to Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 1967. [Google Scholar] [CrossRef] [Green Version]
- Sopo, M.; Anttila, M.; Hämäläinen, K.; Kivelä, A.; Ylä-Herttuala, S.; Kosma, V.M.; Keski-Nisula, L.; Sallinen, H. Expression profiles of VEGF-A, VEGF-D and VEGFR1 are higher in distant metastases than in matched primary high grade epithelial ovarian cancer. BMC Cancer 2019, 19, 584. [Google Scholar] [CrossRef] [Green Version]
- Patel, C.M.; Sahdev, A.; Reznek, R.H. CT, MRI and PET imaging in peritoneal malignancy. Cancer Imaging 2011, 11, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Krishnaraju, V.S.; Kumar, R.; Mittal, B.R.; Sharma, V.; Singh, H.; Nada, R.; Bal, A.; Rohilla, M.; Singh, H.; Rana, S.S. Differentiating benign and malignant pancreatic masses: Ga-68 PSMA PET/CT as a new diagnostic avenue. Eur. Radiol. 2021, 31, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Fujita, Y.; Kato, T.; Horie, K.; Koie, T.; Umezawa, K.; Tsumoto, H.; Miura, Y.; Katagiri, Y.; Miyazaki, T.; et al. Diagnostic potential of serum extracellular vesicles expressing prostate-specific membrane antigen in urologic malignancies. Sci. Rep. 2021, 11, 15000. [Google Scholar] [CrossRef] [PubMed]



| Parameter | “Low/High” PSMA n = 34 | “Medium” PSMA n = 46 | p-Value |
|---|---|---|---|
| Age (median, years) | 62.5 IQR (52.4–70.3) | 60.4 IQR (51.1–66.4) | 0.65 1 |
| FIGO stage | 0.83 2 | ||
| III | 26 | 33 | |
| IV | 8 | 13 | |
| Residual disease | 1.00 2 | ||
| none | 18 | 25 | |
| any | 16 | 21 | |
| Vessel density | 0.81 3 | ||
| low | 3 | 2 | |
| medium | 11 | 15 | |
| high | 20 | 29 | |
| Treatment response after chemotherapy 4 (complete and partial response) | 0.34 2 | ||
| yes | 27 | 42 | |
| no | 2 | 1 | |
| n = 80 | Progression-Free Survival | |||
|---|---|---|---|---|
| 64 Events | Univariate | Multivariate | ||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.02 (0.10–1.04) | 0.107 | 1.01 (0.99–1.04) | 0.310 |
| FIGO stage (IV > III) | 2.18 (1.25–3.83) | 0.006 | 2.07 (1.14–3.74) | 0.016 |
| Residual disease (any > none) | 3.20 (1.89–5.43) | <0.001 | 3.37 (1.89–6.00) | <0.001 |
| PSMA expression (“low/high” > “medium”) | 1.61 (0.98–2.64) | 0.059 | 2.24 (1.32–3.82) | 0.003 |
| n = 80 | Overall Survival | |||
|---|---|---|---|---|
| 42 Events | Univariate | Multivariate | ||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.04 (1.01–1.07) | 0.013 | 1.03 (1.00–1.06) | 0.066 |
| FIGO stage (IV > III) | 1.16 (0.58–2.32) | 0.681 | 1.23 (0.60–2.54) | 0.570 |
| Residual disease (any > none) | 3.14 (1.67–5.87) | <0.001 | 3.71 (1.91–7.20) | <0.001 |
| PSMA expression (“low/high” > “medium”) | 2.08 (1.12–3.87) | 0.020 | 2.73 (1.41–5.29) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofstetter, G.; Grech, C.; Pils, D.; Pammer, J.; Neudert, B.; Pötsch, N.; Baltzer, P.; Traub-Weidinger, T.; Seebacher, V.; Aust, S. Prostate-Specific Membrane Antigen (PSMA) Expression in Tumor-Associated Neovasculature Is an Independent Prognostic Marker in Patients with Ovarian Cancer. J. Pers. Med. 2022, 12, 551. https://doi.org/10.3390/jpm12040551
Hofstetter G, Grech C, Pils D, Pammer J, Neudert B, Pötsch N, Baltzer P, Traub-Weidinger T, Seebacher V, Aust S. Prostate-Specific Membrane Antigen (PSMA) Expression in Tumor-Associated Neovasculature Is an Independent Prognostic Marker in Patients with Ovarian Cancer. Journal of Personalized Medicine. 2022; 12(4):551. https://doi.org/10.3390/jpm12040551
Chicago/Turabian StyleHofstetter, Gerda, Christina Grech, Dietmar Pils, Johannes Pammer, Barbara Neudert, Nina Pötsch, Pascal Baltzer, Tatjana Traub-Weidinger, Veronika Seebacher, and Stefanie Aust. 2022. "Prostate-Specific Membrane Antigen (PSMA) Expression in Tumor-Associated Neovasculature Is an Independent Prognostic Marker in Patients with Ovarian Cancer" Journal of Personalized Medicine 12, no. 4: 551. https://doi.org/10.3390/jpm12040551
APA StyleHofstetter, G., Grech, C., Pils, D., Pammer, J., Neudert, B., Pötsch, N., Baltzer, P., Traub-Weidinger, T., Seebacher, V., & Aust, S. (2022). Prostate-Specific Membrane Antigen (PSMA) Expression in Tumor-Associated Neovasculature Is an Independent Prognostic Marker in Patients with Ovarian Cancer. Journal of Personalized Medicine, 12(4), 551. https://doi.org/10.3390/jpm12040551