Surgical Ventricular Restoration for Ischemic Heart Failure: A Glance at a Real-World Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Echocardiography
2.4. Follow-Up
2.5. Surgical Technique
2.6. Statistical Analysis
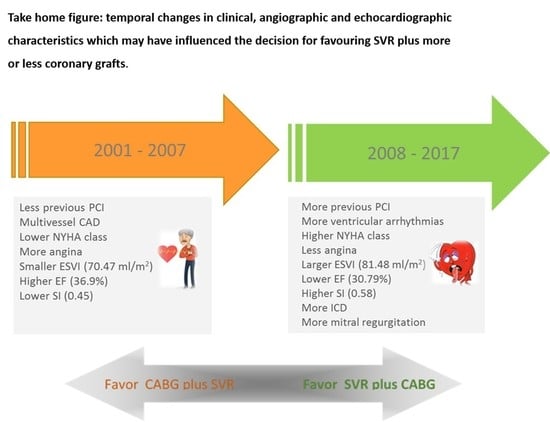
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Sopko, G.; De Luca, L.; Velazquez, E.J.; Parker, J.D.; Binkley, P.F.; Sadowski, Z.; Golba, K.S.; Prior, D.L.; Rouleau, J.L.; et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation 2006, 114, 1202–1213. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Kaul, P.; Bakal, J.A.; Armstrong, P.W.; Welsh, R.C.; McAlister, F.A. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J. Am. Coll. Cardiol. 2009, 53, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. STICHES Investigators. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Velazquez, E.J.; She, L.; Smith, P.K.; Nicolau, J.C.; Favaloro, R.R.; Gradinac, S.; Chrzanowski, L.; Prabhakaran, D.; Howlett, J.G.; et al. Extent of coronary and myocardial disease and benefit from surgical revascularization in ischemic LV dysfunction [Corrected]. J. Am. Coll. Cardiol. 2014, 64, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.H.; Velazquez, E.J.; Michler, R.E.; Sopko, G.; Oh, J.K.; O’Connor, C.M.; Hill, J.A.; Menicanti, L.; Sadowski, Z.; Desvigne-Nickens, P.; et al. STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N. Engl. J. Med. 2009, 360, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Castelvecchio, S.; Pappalardo, O.A.; Menicanti, L. Myocardial reconstruction in ischaemic cardiomyopathy. Eur. J. Cardiothorac. Surg. 2019, 55, i49–i56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Donato, M.; Dabic, P.; Castelvecchio, S.; Santambrogio, C.; Brankovic, J.; Collarini, L.; Joussef, T.; Frigiola, A.; Buckberg, G.; Menicanti, L.; et al. Left ventricular geometry in normal and post-anterior myocardial infarction patients: Sphericity index and ‘new’ conicity index comparisons. Eur. J. Cardiothorac. Surg. 2006, 29, S225–S230. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Harrel, J.R. Regression Modeling Strategies with Applications to Linear Models, Logistic and Ordinal Regression and Survival Analysis; Springer: New York, NY, USA, 2001. [Google Scholar]
- Michler, R.E.; Rouleau, J.L.; Al-Khalidi, H.R.; Bonow, R.O.; Pellikka, P.A.; Pohost, G.M.; Holly, T.A.; Oh, J.K.; Dagenais, F.; Milano, C.; et al. STICH Trial Investigators. Insights from the STICH trial: Change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J. Thorac. Cardiovasc. Surg. 2013, 146, 1139–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelvecchio, S.; Moroni, F.; Menicanti, L. The matter of reverse ventricular remodeling after acute myocardial infarction between fiction and reality. J. Cardiovasc. Med. (Hagerstown) 2018, 19, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Meta-analysis Research Group in Echocardiography (MeRGE) Heart Failure Collaborators; Doughty, R.N.; Klein, A.L.; Poppe, K.K.; Gamble, G.D.; Dini, F.L.; Møller, J.E.; Quintana, M.; Yu, C.M.; Whalley, G.A. Independence of restrictive filling pattern and LV ejection fraction with mortality in heart failure: An individual patient meta-analysis. Eur. J. Heart Fail. 2008, 10, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Rigolli, M.; Rossi, A.; Quintana, M.; Klein, A.L.; Yu, C.M.; Ghio, S.; Dini, F.L.; Prior, D.; Troughton, R.W.; Temporelli, P.L.; et al. The prognostic impact of diastolic dysfunction in patients with chronic heart failure and post-acute myocardial infarction: Can age-stratified E/A ratio alone predict survival? Int. J. Cardiol. 2015, 181, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.O.; Daly, R.C.; Lin, G.; Lahr, B.D.; Wiste, H.J.; Beaver, T.M.; Iacovoni, A.; Malinowski, M.; Friedrich, I.; Rouleau, J.L.; et al. Impact of surgical ventricular reconstruction on sphericity index in patients with ischemic cardiomyopathy: Follow-up from the STICH trial. Eur. J. Heart Fail. 2015, 17, 453–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelvecchio, S.; Menicanti, L.; Ranucci, M.; Di Donato, M. Impact of surgical ventricular restoration on diastolic function: Implications of shape and residual ventricular size. Ann. Thorac. Surg. 2008, 86, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Fatehi Hassanabad, A.; Wiebe, K.; Ali, I.S. Clinical and hemodynamic outcomes of the Dor procedure in adults with ischemic cardiomyopathy. J. Card. Surg. 2021, 36, 4345–4436. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Castelvecchio, S.; Rahouma, M.; Robinson, N.B.; Audisio, K.; Soletti, G.J.; Garatti, A.; Benedetto, U.; Girardi, L.N.; Menicanti, L. Results of surgical ventricular reconstruction in a specialized center and in comparison to the STICH trial: Rationale and study protocol for a patient-level pooled analysis. J. Card. Surg. 2021, 36, 689–692. [Google Scholar] [CrossRef] [PubMed]




| n | Total | n | 2001–2007 (n = 371) | n | 2008–2017 | p-Value | |
|---|---|---|---|---|---|---|---|
| Age, years | 648 | 66 [58–72] | 371 | 67 [58–72] | 277 | 64 [58–71] | 0.1365 |
| BSA | 648 | 1.83 [1.74–1.92] | 371 | 1.83 [1.73–1.94] | 277 | 1.85 [1.75–1.94] | 0.0689 |
| Creatinine | 648 | 1.10 [0.91–1.39] | 371 | 1.14 [0.96–1.44] | 277 | 1.05 [0.88–1.30] | 0.0020 |
| Family history of CAD | 648 | 271 (41.82) | 132 (35.58) | 139 (50.18) | 0.0002 | ||
| Smokers or ex-smokers | 648 | 439 (67.75) | 222 (59.84) | 217 (78.34) | <0.0001 | ||
| Hypertension | 648 | 381 (58.80) | 203 (54.72) | 178 (64.26) | 0.0146 | ||
| Atrial fibrillation | 648 | 91 (14.04) | 49 (13.21) | 42 (15.16) | 0.4786 | ||
| Stroke | 648 | 55 (8.49) | 42 (11.32) | 13 (4.69) | 0.0027 | ||
| Angina | 648 | 230 (35.49) | 176 (47.44) | 54 (19.49) | <0.0001 | ||
| Ventricular arrhythmias | 648 | 106 (16.36) | 31 (8.36) | 75 (27.08) | <0.0001 | ||
| Chronic renal failure | 648 | 47 (7.25) | 24 (6.47) | 23 (8.30) | 0.3731 | ||
| Diabetes mellitus | 648 | 166 (25.66) | 97 (26.15) | 69 (25.00) | 0.7414 | ||
| Hypercholesterolemia | 648 | 381 (58.80) | 188 (50.67) | 193 (69.68) | <0.0001 | ||
| NYHA class III/IV | 648 | 330 (50.93) | 172 (46.36) | 158 (57.04) | 0.0071 | ||
| Previous PCI | 183 (28.24) | 79 (21.29) | 104 (37.55) | <0.0001 | |||
| PCI + ICD | 37 (5.21) | 3 (0.81) | 34 (12.27) | ||||
| ICD | 40 (6.17) | 15 (4.04) | 25 (9.03) | ||||
| ACE inhibitor | 644 | 532 (82.61) | 304 (83.20) | 225 (81.82) | 0.6477 | ||
| β-blockers | 644 | 482 (74.84) | 237 (64.23) | 245 (89.09) | <0.0001 | ||
| Aspirin | 644 | 525 (81.52) | 268 (72.63) | 257 (93.45) | <0.0001 | ||
| Digoxin | 644 | 46 (7.14) | 37 (10.03) | 9 (3.27) | 0.0010 | ||
| Statins | 645 | 434 (67.29) | 176 (47.70) | 258 (93.48) | <0.0001 | ||
| Diuretic | 645 | 537 (83.26) | 280 (75.88) | 257 (93.12) | <0.0001 | ||
| Oral anticoagulant | 644 | 73 (11.34) | 25 (6.79) | 48 (11.39) | <0.0001 | ||
| Amiodarone | 644 | 146 (22.67) | 85 (23.04) | 61 (22.18) | 0.7981 | ||
| Nitrates | 644 | 205 (31.83) | 158 (42.82) | 47 (17.09) | <0.0001 |
| N | Total | n | 2001–2007 (n = 371) | n | 2008–2017 (n = 277) | p-Value | |
|---|---|---|---|---|---|---|---|
| Diastolic diameter (mm) | 630 | 65 [58–71] | 356 | 65 [58–70.50] | 274 | 64 [59–71] | 0.8698 |
| Systolic diameter (mm) | 625 | 51 [44–59] | 355 | 51 [44–59] | 270 | 51 [45–59] | 0.6611 |
| EDV index (mL/m2) | 645 | 112.12 [92.34–134.30] | 368 | 106.68 [88.77–128.06] | 277 | 118.27 [98.86–140.83] | <0.0001 |
| ESV index (mL/m2) | 646 | 76.08 [59.17–95.81] | 369 | 70.47 [55.65–92.02] | 277 | 81.48 [65.79–101.85] | <0.0001 |
| EF (%) | 648 | 32 [26–37] | 371 | 33 [26–38] | 277 | 31 [25–36] | 0.0100 |
| SV index (mL/m2) | 644 | 35.20 [29.38–41.69] | 367 | 34.69 [24.14–40.61] | 277 | 36.57 [29.63–42.55] | 0.1186 |
| TAPSE (mm) | 609 | 20 [18–23] | 335 | 20 [17–23] | 274 | 21 [18–24] | 0.0158 |
| PAPs (mmHg) | 556 | 38 [30.5–48] | 282 | 38 [32–46] | 274 | 38 [30–49] | 0.4370 |
| LVMI (g/m2) | 561 | 164.29 [137.88–199.48] | 336 | 168.26 [141.93–203.81] | 225 | 160.51 [131.22–193.68] | 0.0060 |
| RWT | 597 | 0.32 [0.27–0.38] | 347 | 0.33 [0.28–0.40] | 250 | 0.29 [0.25–0.36] | <0.0001 |
| Left atrial diameter (mm) | 594 | 46 [41–51] | 340 | 46 [40.50–50] | 254 | 47 [43–52] | 0.0083 |
| E/A ratio | 458 | 0.96 [0.66–1.71] | 220 | 0.82 [0.64–1.40] | 238 | 1.15 [0.72–2.09] | 0.0003 |
| DT (mm) | 435 | 185 [149–239] | 197 | 190 [155–231] | 238 | 182 [144–254] | 0.9478 |
| Mitral annulus (mm) | 367 | 34 [30–37] | 162 | 35 [31–39] | 205 | 32 [29–37] | 0.0017 |
| IPD, diastole (mm) | 254 | 3 [2.5–3.5] | 124 | 2.60 [2.20–3.1] | 132 | 3.20 [2.8–3.6] | <0.0001 |
| IPD, systole (mm) | 254 | 2.10 [1.7–2.6] | 124 | 1.77 [1.4–2.6] | 132 | 2.30 [2.00–2.7] | <0.0001 |
| Sphericity index, diastole | 395 | 0.60 [0.50–0.68] | 152 | 0.60 [0.50–0.68] | 243 | 0.64 [0.57–0.72] | <0.0001 |
| Sphericity index, systole | 394 | 0.53 [0.43–0.62] | 152 | 0.45 [0.37–0.54] | 243 | 0.58 [0.49–0.65] | <0.0001 |
| Conicity index, diastole | 385 | 0.88 [0.80–0.96] | 142 | 0.81 [0.73–0.95] | 243 | 0.89 [0.83–0.97] | <0.0001 |
| Conicity index, systole | 385 | 0.94 [0.83–1.09] | 142 | 0.95 [0.80–1.11] | 243 | 0.93 [0.84–1.08] | 0.8379 |
| MR grade | |||||||
| 0 | 80 (12.35) | 74 (19.95) | 6 (2.17) | ||||
| 1 | 209 (32.25) | 126 (33.96) | 83 (29.96) | ||||
| 2 | 142 (21.91) | 66 (17.79) | 76 (27.44) | <0.0001 | |||
| 3 | 119 (18.36) | 58 (15.63) | 61 (22.02) | ||||
| 4 | 98 (15.12) | 47 (12.67) | 51 (18.41) | ||||
| Site of remodeling | |||||||
| Posterior | 96 (14.81) | 44 (11.86) | 52 (18.77) | ||||
| Anterior | 526 (81.17) | 316 (85.18) | 210 (75.81) | 0.0100 | |||
| Anterior & posterior | 26 (4.01) | 11 (2.96) | 15 (5.42) | ||||
| Coronary angiography | |||||||
| Single vessel disease | 159 (24.54) | 85 (22.91) | 74 (26.71) | ||||
| Multivessel disease | 436 (67.28) | 272 (73.32) | 164 (59.21) | <0.0001 | |||
| No residual stenosis | 53 (8.18) | 14 (3.77) | 39 (14.08) | ||||
| Mitral valve surgery | 200 (30.86) | 96 (25.88) | 104 (37.55) | 0.0015 | |||
| CABG + SVR | 582 (89.81) | 347 (93.53) | 235 (84.84) | 0.0003 | |||
| SVR | 66 (10.19) | 24 (6.47) | 42 (15.16) | ||||
| Number of distal anastomosis | |||||||
| 0 | 66 (10.19) | 24 (6.47) | 42 (15.16) | ||||
| 1 | 144 (22.22) | 69 (18.60) | 75 (27.08) | <0.0001 | |||
| >=2 | 438 (67.59) | 278 (74.93) | 160 (57.76) |
| 2001–2007 (n = 371) | 2008–2017 (n = 277) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| Death (n = 212) | Death | Death (n = 57) | Death | ||||||
| Risk Category | Hazard Ratio (95% IC) | p-Value | Hazard Ratio (95% IC) | p-Value | Hazard Ratio (95% IC) | p-Value | Hazard Ratio (95% IC) | p-Value | |
| Sex | M vs. F | 0.83 [0.60–1.17] | 0.2899 | 0.94 [0.44–1.99] | 0.8688 | ||||
| Site of remodeling | |||||||||
| Ant/post | Ant/post vs. Ant | 1.82 [0.89–3.71] | 0.0991 | 2.61 [1.17–5.85] | 0.0194 | ||||
| Post | Post vs. Ant | 1.52 [1.03–2.24] | 0.0359 | 1.56 [0.79–3.07] | 0.1996 | ||||
| Previous MI | Yes vs. No | 1.10 [0.76–1.59] | 0.5986 | 1.48 [0.73–3.03] | 0.2761 | ||||
| Hypertension | Yes vs. No | 1.04 [0.79–1.36] | 0.7783 | 1.01 [0.60–1.73] | 0.9739 | ||||
| Dyslipidemia | Yes vs. No | 0.66 [0.50–0.86] | 0.0024 | 1.06 [0.60–1.87] | 0.8449 | ||||
| Diabetes | Yes vs. No | 1.24 [0.92–1.66] | 0.1578 | 1.96 [1.15–3.34] | 0.0136 | 1.96 [0.94–4.08] | 0.0728 | ||
| Smoke | Yes vs. No | 1.18 [0.90–1.56] | 0.2340 | 0.92 [0.50–1.71] | 0.7928 | ||||
| Previous procedures | |||||||||
| PCI vs. No | 0.87 [0.61–1.24] | 0.4436 | 0.57 [0.30–1.09] | 0.0908 | |||||
| PCI + ICD vs. No | 2.27 [0.56–9.20] | 0.2493 | 1.33 [0.60–2.96] | 0.4868 | |||||
| ICD vs. No | 1.01 [0.49–2.05] | 0.9849 | 1.79 [0.81–3.99] | 0.1516 | |||||
| Other vs. No | 1.48 [0.92–2.40] | 0.1071 | 0.98 [0.14–7.22] | 0.9831 | |||||
| Ventricular arrhythmias | Yes vs. No | 1.29 [0.81–2.04] | 0.2842 | 0.758 [0.40–1.43] | 0.3858 | ||||
| Atrial fibrillation | Yes vs. No | 1.47 [1.02–2.11] | 0.0384 | 3.14 [1.79–5.49] | <0.0001 | ||||
| Stroke | Yes vs. No | 1.32 [0.88–1.99] | 0.1864 | 1.63 [0.59–4.50] | 0.3496 | ||||
| Chronic renal failure | Yes vs. No | 2.92 [1.87–4.56] | <0.0001 | 3.36 [1.74–6.50] | 0.0003 | ||||
| Angiography | |||||||||
| Single vessels | Single vessels vs. | 0.67 [0.33–1.37] | 0.2750 | 0.96 [0.35–2.59] | 0.9317 | 0.91 [0.31–2.67] | 0.8617 | ||
| No significant stenosis | |||||||||
| Multivessel disease | Multivessels disease vs. | 0.95 [0.48–1.86] | 0.8761 | 1.63 [0.62–4.28] | 0.3219 | 1.68 [0.66–4.27] | 0.2728 | ||
| No significant stenosis | |||||||||
| Angina | Yes vs. No | 1.20 [0.91–1.57] | 0.1885 | 0.83 [0.44–1.58] | 0.5709 | ||||
| NYHA | III-IV vs. I-II | 1.82 [1.38–2.39] | <0.0001 | 3.12 [1.65–5.90] | 0.0005 | 4.28 [1.47–12.44] | 0.0075 | ||
| MR grade | >= 2 vs. <2 | 1.41 [1.08–1.85] | 0.0126 | 2.48 [1.25–4.92] | 0.0092 | ||||
| Age | 1 unit | 1.06 [1.04–1.07] | <0.0001 | 1.07 [1.05–1.09] | <0.0001 | 1.07 [1.03–1.10] | <0.0001 | 1.04 [0.99–1.09] | 0.0815 |
| BSA | 1 unit | 0.96 [0.43–2.14] | 0.9241 | 0.07 [0.01–0.54] | 0.0099 | ||||
| Haemoglobin | 1 unit | 0.86 [0.78–0.94] | 0.0005 | 0.83 [0.70–0.97] | 0.0205 | ||||
| Creatinine | 1 unit | 2.15 [1.78–2.59] | <0.0001 | 1.62 [1.21–2.18] | 0.0012 | ||||
| Diastolic diameter (mm) | 1 unit | 1.02 [1.00–1.04] | 0.0126 | 1.03 [1.00–1.06] | 0.0511 | ||||
| Systolic diameter (mm) | 1 unit | 1.02 [1.00–1.03] | 0.0122 | 1.03 [1.00–1.06] | 0.0173 | ||||
| EDV index (mL/m2) | 1 unit | 1.00 [1.00–1.01] | 0.0906 | 1.00 [1.00–1.01] | 0.4235 | ||||
| ESV index (mL/m2) | 1 unit | 1.00 [1.00–1.01] | 0.0070 | 1.00 [1.00–1.01] | 0.1935 | ||||
| EF | 1 unit | 0.96 [0.94–0.98] | <0.0001 | 0.97 [0.95–0.99] | 0.0151 | 0.95 [0.92–0.96] | 0.0051 | ||
| SV index (mL/m2) | 1 unit | 0.98 [0.97–0.99] | 0.0421 | 0.98 [0.95–1.01] | 0.2228 | ||||
| RWT | 1 unit | 0.11 [0.03–0.53] | 0.0054 | 0.09 [0.01–1.29] | 0.0765 | ||||
| LVMI (g/m2) | 1 unit | 1.00 [1.00–1.00] | 0.2207 | 1.00 [1.00–1.01] | 0.1060 | ||||
| Left atrial diameter (mm) | 1 unit | 1.04 [1.02–1.06] | <0.0001 | 1.05 [1.01–1.09] | 0.0146 | ||||
| E/A ratio | 1 unit | 1.50 [1.23–1.84] | <0.0001 | 1.61 [1.31–1.99] | <0.0001 | 1.39 [1.09–1.77] | 0.0079 | ||
| DT (m sec) | 1 unit | 0.99 [0.99–1.00] | 0.0604 | 1.00 [0.99–1.00] | 0.2290 | ||||
| TAPSE | 1 unit | 0.94 [0.91–0.98] | 0.0027 | 0.91 [0.84–0.97] | 0.0053 | ||||
| PAPs (mmHg) | 1 unit | 1.02 [1.01–1.03] | 0.0006 | 1.02 [1.01–1.04] | 0.0082 | ||||
| Mitral annulus (mm) | 1 unit | 1.05 [1.02–1.09] | 0.0051 | 1.05 [0.99–1.11] | 0.0800 | ||||
| IPD, diastole (mm) | 1 unit | 1.36 [1.09–1.68] | 0.0058 | 1.34 [0.65–2.77] | 0.4312 | ||||
| IPD, systole (mm) | 1 unit | 1.47 [1.18–1.84] | 0.0007 | 1.68 [0.89–3.17] | 0.1061 | ||||
| MR | Yes vs. No | 1.73 [1.30–2.30] | 0.0002 | 2.64 [1.56–4.47] | 0.0003 | ||||
| Diastolic sphericity index | 1 unit | 1.01 [0.99–1.03] | 0.4265 | 1.04 [1.01–1.07] | 0.0104 | 1.03 [1.00–1.07] | 0.0379 | ||
| Systolic sphericity index | 1 unit | 1.02 [1.00–1.03] | 0.0931 | 1.04 [1.01–1.07] | 0.0041 | ||||
| Diastolic conicity index | 1 unit | 0.99 [0.98–1.00] | 0.1062 | 0.98 [0.96–1.01] | 0.1311 | ||||
| Systolic conicity index | 1 unit | 0.99 [0.99–1.00] | 0.1099 | 0.99 [0.97–1.00] | 0.0552 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelvecchio, S.; Milani, V.; Ambrogi, F.; Volpe, M.; Ramputi, L.; Soletti, G., Jr.; Menicanti, L. Surgical Ventricular Restoration for Ischemic Heart Failure: A Glance at a Real-World Population. J. Pers. Med. 2022, 12, 567. https://doi.org/10.3390/jpm12040567
Castelvecchio S, Milani V, Ambrogi F, Volpe M, Ramputi L, Soletti G Jr., Menicanti L. Surgical Ventricular Restoration for Ischemic Heart Failure: A Glance at a Real-World Population. Journal of Personalized Medicine. 2022; 12(4):567. https://doi.org/10.3390/jpm12040567
Chicago/Turabian StyleCastelvecchio, Serenella, Valentina Milani, Federico Ambrogi, Marianna Volpe, Lucia Ramputi, Giovanni Soletti, Jr., and Lorenzo Menicanti. 2022. "Surgical Ventricular Restoration for Ischemic Heart Failure: A Glance at a Real-World Population" Journal of Personalized Medicine 12, no. 4: 567. https://doi.org/10.3390/jpm12040567
APA StyleCastelvecchio, S., Milani, V., Ambrogi, F., Volpe, M., Ramputi, L., Soletti, G., Jr., & Menicanti, L. (2022). Surgical Ventricular Restoration for Ischemic Heart Failure: A Glance at a Real-World Population. Journal of Personalized Medicine, 12(4), 567. https://doi.org/10.3390/jpm12040567