Atrial Fibrillation Specific Exercise Rehabilitation: Are We There Yet?
Abstract
:1. Why Do We Need AF-Specific, Exercise-Based Rehabilitation?
2. Methods
3. Evidence Informing AF-Specific Rehabilitation
3.1. Study Characteristics
3.2. Intervention/Cohort Details and Long-Term Outcomes
3.3. Should We Promote Increased Intensity or Duration of Exercise for Improved Outcomes?
3.4. Mechanisms of AF Protection from Long-Term Exercise Training
3.5. Is There an Acute, AF-Specific, Cardioprotective Effect of Exercise for People with AF?
4. Limitations
5. Perspectives of Future Directions
6. Conclusions
- Four systematic reviews and 21 primary studies were included that investigated exercise training or physical activity for people with AF.
- Included studies represented a range of interventions and participant characteristics including females, males, and all AF subtypes.
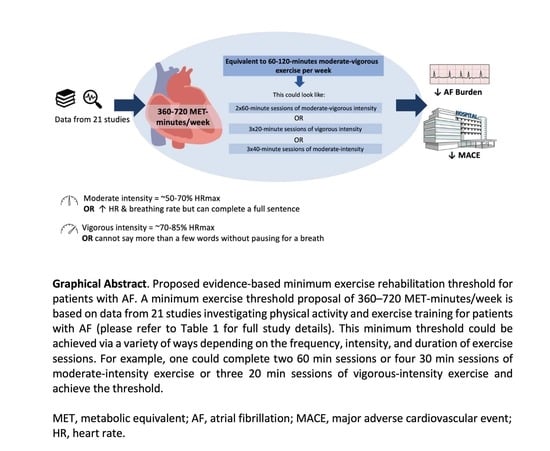
- We propose 360–720 MET-minutes/week, corresponding to ~60–120 min of moderate-vigorous intensity exercise per week as an evidence-based recommendation for patients with AF.
- This recommendation could be achieved in a variety of ways to suit the individual i.e., two 60 min or four 30 min sessions of moderate-intensity exercise or three 20 min sessions of high-intensity exercise, for example.
- Further work is needed to explore the potential of immediate effects of lifestyle-related risk factors, such as physical activity and sleep quality, on AF recurrence and burden.
- Furthermore, adequately powered RCTs are needed to investigate the effectiveness of exercise-based rehabilitation on AF-specific outcome measures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dibben, G.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.-D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2021, 11, 1465–1858. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Thijssen, D.H.J.; Lip, G.Y.H. Exercise-Based Cardiac Rehabilitation and All-Cause Mortality Among Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e020804. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Sankaranarayanan, R.; Wright, D.J.; Thijssen, D.H.J.; Lip, G.Y.H. Cardiac rehabilitation and all-cause mortality in patients with heart failure: A retrospective cohort study. Eur. J. Prev. Cardiol. 2021, 28 (Suppl. 1), 1704–1710. [Google Scholar] [CrossRef]
- Anderson, L.; Taylor, R. Cardiac rehabilitation for people with heart disease: An overview of Cochrane systematic reviews. Int. J. Cardiol. 2014, 177, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.-W.; Kim, S.-H.; Kang, S.-H.; Kim, H.-J.; Yoon, C.-H.; Youn, T.-J.; Chae, I.-H. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur. Heart J. 2019, 40, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise: One size does not fit all. J. Physiol. 2020, 598, 3819–3820. [Google Scholar] [CrossRef]
- Gevaert, A.; Adams, V.; Bahls, M.; Bowen, T.S.; Cornelissen, V.; Dörr, M.; Hansen, D.; Mc Kemps, H.; Leeson, P.; Van Craenenbroeck, E.M.; et al. Towards a personalised approach in exercise-based cardiovascular rehabilitation: How can translational research help? A ‘call to action’ from the Section on Secondary Prevention and Cardiac Rehabilitation of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2020, 27, 1369–1385. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Medicine ACoS. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Risom, S.S.; Zwisler, A.-D.; Johansen, P.P.; Sibilitz, K.L.; Lindschou, J.; Gluud, C.; Taylor, R.S.; Svendsen, J.H.; Berg, S.K. Exercise-based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst. Rev. 2017, 2, CD011197. [Google Scholar] [CrossRef] [Green Version]
- Smart, N.A.; King, N.; Lambert, J.D.; Pearson, M.J.; Campbell, J.; Risom, S.S.; Taylor, R.S. Exercise-based cardiac rehabilitation improves exercise capacity and health-related quality of life in people with atrial fibrillation: A systematic review and meta-analysis of randomised and non-randomised trials. Open Heart 2018, 5, e000880. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.L.; Terada, T.; Chirico, D.; Prince, S.A.; Pipe, A.L. The Effects of Cardiac Rehabilitation in Patients With Atrial Fibrillation: A Systematic Review. Can. J. Cardiol. 2018, 34 (Suppl. 2), S284–S295. [Google Scholar] [CrossRef]
- Elkhair, A.A.; Barraclough, D.; Boidin, M.; Buckley, B.; Lane, D.; Lip, G.; Thijssen, D.; Williams, N. Effect of Different Types of Exercise on Quality of Life for Patients with Atrial Fibrillation: Systematic Review. Prospero ID CRD42021231102. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=231102 (accessed on 10 February 2022).
- Buckley, B.; Lip, G.Y.H.; Thijssen, D.H.J. The counterintuitive role of exercise in the prevention and cause of atrial fibrillation. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1051–H1058. [Google Scholar] [CrossRef]
- Luo, N.; Merrill, P.; Parikh, K.S.; Whellan, D.J.; Piña, I.L.; Fiuzat, M.; Kraus, W.E.; Kitzman, D.W.; Keteyian, S.J.; O’Connor, C.M.; et al. Exercise Training in Patients With Chronic Heart Failure and Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 69, 1683–1691. [Google Scholar] [CrossRef]
- Malmo, V.; Nes, B.M.; Amundsen, B.H.; Tjonna, A.-E.; Stoylen, A.; Rossvoll, O.; Wisloff, U.; Loennechen, J.P. Aerobic Interval Training Reduces the Burden of Atrial Fibrillation in the Short Term: A Randomized Trial. Circulation 2016, 133, 466–473. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Lee, S.-R.; Choi, E.-K.; Han, K.-D.; Jung, J.-H.; Lim, J.-H.; Yun, J.-P.; Kwon, S.; Oh, S.; Lip, G.Y.H. Association between exercise habits and stroke, heart failure, and mortality in Korean patients with incident atrial fibrillation: A nationwide population-based cohort study. PLoS Med. 2021, 18, e1003659. [Google Scholar] [CrossRef]
- Melo, X.; Abreu, A.; Santos, V.; Cunha, P.; Oliveira, M.; Pinto, R.; Carmo, M.; Fernhall, B.; Santa-Clara, H. A Post hoc analysis on rhythm and high intensity interval training in cardiac resynchronization therapy. Scand. Cardiovasc. J. 2019, 53, 197–205. [Google Scholar] [CrossRef]
- Hegbom, F.; Stavem, K.; Sire, S.; Heldal, M.; Orning, O.M.; Gjesdal, K. Effects of short-term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int. J. Cardiol. 2007, 116, 86–92. [Google Scholar] [CrossRef]
- Rienstra, M.; Hobbelt, A.H.; Alings, M.; Tijssen, J.G.P.; Smit, M.D.; Brügemann, J.; Geelhoed, B.; Tieleman, R.G.; Hillege, H.L.; Tukkie, R.; et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: Results of the RACE 3 trial. Eur. Heart J. 2018, 39, 2987–2996. [Google Scholar] [CrossRef]
- Risom, S.S.; Zwisler, A.-D.; Rasmussen, T.B.; Sibilitz, K.L.; Madsen, T.L.; Svendsen, J.H.; Gluud, C.; Lindschou, J.; Winkel, P.; Berg, S.K. Cardiac rehabilitation versus usual care for patients treated with catheter ablation for atrial fibrillation: Results of the randomized CopenHeartRFA trial. Am. Heart J. 2016, 181, 120–129. [Google Scholar] [CrossRef]
- Risom, S.S.; Zwisler, A.-D.; Sibilitz, K.L.; Rasmussen, T.B.; Taylor, R.S.; Thygesen, L.C.; Madsen, T.S.; Svendsen, J.H.; Berg, S.K. Cardiac Rehabilitation for Patients Treated for Atrial Fibrillation With Ablation Has Long-Term Effects: 12-and 24-Month Follow-up Results From the Randomized CopenHeart(RFA) Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Skielboe, A.K.; Bandholm, T.Q.; Hakmann, S.; Mourier, M.; Kallemose, T.; Dixen, U. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation—A randomized controlled trial. PLoS ONE 2017, 12, e0170060. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, M.; Rosenqvist, M.; Medin, J.; Walfridsson, U.; Rydell-Karlsson, M. MediYoga as a part of a self-management programme among patients with paroxysmal atrial fibrillation—A randomised study. Eur. J. Cardiovasc. Nur. 2020, 19, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, M.; Karlsson, M.R.; Medin, J.; Frykman, V. Effects of yoga in patients with paroxysmal atrial fibrillation—A randomized controlled study. Eur. J. Cardiovasc. Nur. 2017, 16, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Joensen, A.M.; Dinesen, P.T.; Svendsen, L.T.; Hoejbjerg, T.K.; Fjerbaek, A.; Andreasen, J.; Sottrup, M.B.; Lundbye-Christensen, S.; Vadmann, H.; Riahi, S. Effect of patient education and physical training on quality of life and physical exercise capacity in patients with paroxysmal or persistent atrial fibrillation: A randomized study. J. Rehabil. Med. 2019, 51, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Ogano, M.; Mori, Y.; Kochi, K.; Morimoto, D.; Kito, K.; Green, F.N.; Tsukamoto, T.; Kubo, A.; Takagi, H.; et al. Exercise-based cardiac rehabilitation for patients with catheter ablation for persistent atrial fibrillation: A randomized controlled clinical trial. Eur. J. Prev. Cardiol. 2019, 26, 1931–1940. [Google Scholar] [CrossRef]
- Buckley, B.; Harrison, S.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.; Thijssen, D.; Lip, G. Association of Exercise-Based Cardiac Rehabilitation with Progression of Paroxysmal to Sustained Atrial Fibrillation. J. Clin. Med. 2021, 10, 435. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Atkins, D.; Pillarisetti, J.; Ryschon, K.; Bommana, S.; Drisko, J.; Vanga, S.; Dawn, B. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: The YOGA My Heart Study. J. Am. Coll. Cardiol. 2013, 61, 1177–1182. [Google Scholar] [CrossRef]
- Osbak, P.S.; Mourier, M.; Kjaer, A.; Henriksen, J.H.; Kofoed, K.F.; Jensen, G.B. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am. Heart J. 2011, 162, 1080–1087. [Google Scholar] [CrossRef]
- Pippa, L.; Manzoli, L.; Corti, I.; Congedo, G.; Romanazzi, L.; Parruti, G. Functional capacity after traditional Chinese medicine (qi gong) training in patients with chronic atrial fibrillation: A randomized controlled trial. Prev. Cardiol. 2007, 10, 22–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeren, M.; Demir, R.; Yigit, Z.; Gürses, H.N. Effects of inspiratory muscle training on pulmonary function, respiratory muscle strength and functional capacity in patients with atrial fibrillation: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Garnvik, L.E.; Malmo, V.; Janszky, I.; Ellekjær, H.; Wisløff, U.; Loennechen, J.P.; Nes, B.M. Physical activity, cardiorespiratory fitness, and cardiovascular outcomes in individuals with atrial fibrillation: The HUNT study. Eur. Heart J. 2020, 41, 1467–1475. [Google Scholar] [CrossRef]
- Bonnesen, M.P.; Frodi, D.M.; Haugan, K.J.; Kronborg, C.; Graff, C.; Højberg, S.; Køber, L.; Krieger, D.; Brandes, A.; Svendsen, J.H.; et al. Day-to-day measurement of physical activity and risk of atrial fibrillation. Eur. Heart J. 2021, 42, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Shaviv, E.; Nof, E.; Israel, A.; Berkovitch, A.; Goldenberg, I.; Glikson, M.; Klempfner, R.; Beinart, R. The role and outcome of cardiac rehabilitation program in patients with atrial fibrillation. Clin. Cardiol. 2018, 41, 1170–1176. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chung, M.K.; Allen, L.A.; Ezekowitz, M.; Furie, K.L.; McCabe, P.; Noseworthy, P.A.; Perez, M.V.; Turakhia, M.P.; American Heart Association Council on Clinical Cardiology; et al. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e623–e644. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; McFarlane, J.R.; Dieberg, G.; Smart, N.A. Exercise training program characteristics and magnitude of change in functional capacity of heart failure patients. Int. J. Cardiol. 2014, 171, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Vromen, T.; Kraal, J.; Kuiper, J.; Spee, R.; Peek, N.; Kemps, H. The influence of training characteristics on the effect of aerobic exercise training in patients with chronic heart failure: A meta-regression analysis. Int. J. Cardiol. 2016, 208, 120–127. [Google Scholar] [CrossRef]
- Williams, C.J.; Gurd, B.J.; Bonafiglia, J.T.; Voisin, S.; Li, Z.; Harvey, N.; Croci, I.; Taylor, J.L.; Gajanand, T.; Ramos, J.S.; et al. A Multi-Center Comparison of O(2peak) Trainability Between Interval Training and Moderate Intensity Continuous Training. Front. Physiol. 2019, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Montero, D.; Lundby, C. Refuting the myth of non-response to exercise training: ‘non-responders’ do respond to higher dose of training. J. Physiol. 2017, 595, 3377–3387. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Gorber, S.C.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, S.; Winzer, E.B.; Duvinage, A.; Gevaert, A.B.; Edelmann, F.; Haller, B.; Pieske-Kraigher, E.; Beckers, P.; Bobenko, A.; Hommel, J.; et al. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2021, 325, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.J.R.; Lip, G.Y.H.; Thijssen, D.H.J. Effect of Training on Peak Oxygen Consumption in Patients with Heart Failure with Preserved Ejection Fraction. JAMA 2021, 326, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Unal, B.; Critchley, J.; Capewell, S. Mortality reductions in patients receiving exercise-based cardiac rehabilitation: How much can be attributed to cardiovascular risk factor improvements? Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Cook, N.; Buring, J.E.; Ridker, P.M.; Lee, I.-M. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms. Circulation 2007, 116, 2110–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, B.J.R.; Boidin, M.; Thijssen, D.H.J. Assessment of Peripheral Blood Flow and Vascular Function. In Sport and Exercise Physiology Testing Guidelines; Taylor & Francis Group: Abingdon, UK, 2022. [Google Scholar]
- Green, D.J.; Hopman, M.T.E.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H.J. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; O’Driscoll, G.; Joyner, M.J.; Cable, N.T. Exercise and cardiovascular risk reduction: Time to update the rationale for exercise? J. Appl. Physiol. 2008, 105, 766–768. [Google Scholar] [CrossRef] [Green Version]
- Thijssen, D.H.J.; Carter, S.; Green, D.J. Arterial structure and function in vascular ageing: Are you as old as your arteries? J. Physiol. 2016, 594, 2275–2284. [Google Scholar] [CrossRef]
- Seo, D.Y.; Kwak, H.-B.; Kim, A.H.; Park, S.H.; Heo, J.W.; Kim, H.K.; Ko, J.R.; Lee, S.J.; Bang, H.S.; Sim, J.W.; et al. Cardiac adaptation to exercise training in health and disease. Pflug. Arch. Eur. J. Physiol. 2020, 472, 155–168. [Google Scholar] [CrossRef]
- Qin, S.; Boidin, M.; Buckley, B.J.R.; Lip, G.Y.H.; Thijssen, D.H.J. Endothelial dysfunction and vascular maladaptation in atrial fibrillation. Eur. J. Clin. Investig. 2021, 51, e13477. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Redington, A.; George, K.P.; Hopman, M.T.E.; Jones, H. Association of Exercise Preconditioning With Immediate Cardioprotection: A Review. JAMA Cardiol. 2018, 3, 169–176. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Uthman, L.; Somani, Y.; van Royen, N. Short term exercise-induced protection of cardiovascular function and health: Why and how fast does the heart benefit from exercise? J. Physiol. 2022, 600, 1339–1355. [Google Scholar] [CrossRef]
- Coumel, P. Paroxysmal atrial fibrillation: A disorder of autonomic tone? Eur. Heart J. 1994, 15 (Suppl. A), 9–16. [Google Scholar] [CrossRef]
- Linz, D.; Brooks, A.G.; Elliott, A.D.; Nalliah, C.J.; Hendriks, J.; Middeldorp, M.; Gallagher, C.; Mahajan, R.; Kalman, J.M.; McEvoy, R.D.; et al. Variability of Sleep Apnea Severity and Risk of Atrial Fibrillation: The VARIOSA-AF Study. JACC Clin. Electrophysiol. 2019, 5, 692–701. [Google Scholar] [CrossRef]
- Lau, Y.C.; Lane, D.A.; Lip, G.Y. Atrial fibrillation in cryptogenic stroke: Look harder, look longer, but just keep looking. Stroke A J. Cereb. Circ. 2014, 45, 3184–3185. [Google Scholar] [CrossRef] [Green Version]

| First Author, Year | AF Population n = Sample; Age (Years); Sex (%); AF Subtype | Exercise Intervention Frequency; Intensity; Time; Type | Follow-Up and Impact on Health Outcomes (SAE, Physical Capacity, AF Specific Outcomes, and QoL) |
|---|---|---|---|
| Randomised Controlled Trials | |||
| Luo, 2017 [17] | n = 382 63 years 16% female AF and heart failure (excluded if sustained fast AF) | Frequency: 3 sessions/week for 36 sessions, followed by transition to a home-based exercise program for 2 years. Intensity: NR Time: 90 min/week for 3 months, followed by 120 min/week thereafter Type: Aerobic exercise (walking, treadmill, or cycle ergometer) | Follow-up: median of 2.6 years SAE: AF was associated with a 24% per year higher rate of mortality/hospitalisation in the control group compared to intervention group (HR: 1.53; 95% CI: 1.34 to 1.74; p < 0.001) in unadjusted analysis; this did not remain significant after adjustment (HR: 1.15;95% CI: 0.98 to 1.35; p > 0.09). No interaction between AF and exercise training (all p > 0.10). No difference in AF event rates between groups (all p > 0.10). |
| Malmo, 2016 [18] | n = 51 Intervention: 56 years 77% female Control: 62 years 88% male Paroxysmal or persistent AF | Frequency: 3 sessions/week for 12 weeks Intensity: Vigorous (85–95% of maximal heart rate and Borg 6–20) Time: 4 min intervals Type: Walking or running on treadmill | Follow-up: 20 weeks Mean time in AF: 10.4% (95% CI, 4.6–17.8) to 14.6% (95% CI, 6.4–24.9) in the control group vs. 8.1% (95% CI, 4.1–12.8) to 4.8% (95% CI, 2.0–7.6) in the exercise group (p = 0.001). V˙O2-peak change to follow-Up: Control group: −0.3 ± 4.3 vs. Exercise group: 3.2 ± 2.5, p < 0.001. Quality of life change to Follow-Up, SF-36: Physical component score: Control group: −0.3 ± 5.4 vs. exercise group: 2.2 ± 4.4 Mental component score: Control group: 1.4 ± 7.2 vs. Exercise group: 3.6 ± 6.5, p > 0.05. No serious adverse events, 2 patients in EG had bursitis episodes, 2 patients in CG had a stroke and ventricular tachycardia. |
| Osbak, 2011 | n = 49 Intervention: 70 years Control: 71 years 75% male Permanent AF | Frequency: 3 sessions/week for 12 weeks Intensity: Vigorous (70% of maximal exercise capacity or Borg scale 14–16/20) Time: 1 h Type: Group-based aerobic training including ergometer cycling, walking on stairs, running, fitness training on physioballs, and interval training. | Follow-up: 12 weeks Physical capacity: 6MWT: Significantly increased within the intervention group (from 504.4 (85.1) m to 569.9 (92.6) m (p < 0.001) and between the groups (EG = 569.9 (92.6) m and CG = 454.1 (95.7), (p = 0.001). QoL: MLHF-Q score: intervention group: 21.1 ± 18.0 vs. control group: 15.4 ± 17.5, p = 0.03. SF-36 subscales: physical functioning (p = 0.02), general health perceptions (p = 0.001), and vitality (p = 0.02) in favour of the intervention group. No adverse events |
| Pippa, 2007 | n = 43 Intervention: 68 years 64% male Control: 68 years 76% male Permanent AF | Frequency: 2 sessions/week for 16 weeks Intensity: NR (predict light) Time: 90 min Type: Qigong training (consists of slow and graceful movements with a focus on breathing) | Follow-up: 16 weeks Physical capacity: 6MWT: Intervention group: 531(121) meters at the end of intervention, 474 (109) meters at 16 weeks after intervention vs. control group: 380 (97) meters at the end of intervention, 350 (110) meters after 16 weeks. p < 0.001. 1 retinal embolism in the intervention and 1 case of deep vein thrombosis during the follow-up in the control group. |
| Rienstra, 2018 | n = 245 Intervention: 64 years 79% male Control: 65 years 79% male Persistent AF | Frequency: 2–3 sessions/week for 9–11 weeks Intensity: NR Time: 20–30 min exercise, Type: Cardiac rehabilitation | Follow-up: 1 year AF specific: At 1 year, sinus rhythm was present in 89 (75%) patients in the intervention vs. 79 (63%) in the conventional group (odds ratio 1.765, lower limit of 95% confidence interval 1.021, p = 0.042). |
| Risom, 2016 Long-term follow up: Risom, 2020 | n = 210 Intervention: 60 years Male 70% Control: 59 years 73% male Paroxysmal or persistent AF | Frequency: 3 sessions/week for 12 weeks Intensity: Intensity was progressively increased Time: 1 h Type: Comprehensive cardiac rehabilitation. Cardiovascular training and strength exercises | Follow-up: 6 and 12-months SAE: Mortality: one death in each group at 6- and 24-months follow-up (p > 0.99). All hospital admissions: Intervention group: n = 71 (68%) vs. control group: n = 60 (57%). Physical capacity: V˙O2-peak at four months: (Intervention group: 24.3 mL kg−1 min−1 vs control group: 20.7 mL kg−1 min−1, p = 0.003). VO2 peak at twelve months: (Intervention group: 25.8 mL kg−1 min−1 vs control group: 22.4 mL kg−1 min−1, p = 0.002). OoL: SF-36: General health Perception (Intervention group: 67.16 points vs. control group: 66.9 points. p = 0.02, of Interaction between Intervention and time). No significant difference between groups was found at six or 24 at the other domains. AFEQT: The results are in favour of the intervention group: Global score: (Intervention group: 81.64 points vs. control group: 82.87 points p = 0.04, of Interaction between Intervention and time) and the treatment satisfaction p = 0.03, of Interaction between Intervention and time score at 24 months. Two serious adverse events (AF intervention- related and unrelated to intervention death in the EG and 1 unrelated to intervention death in the CG group. 16 non-serious adverse events EG and 7 in the CG. |
| Skielboe, 2017 | n = 76 Low intensity: 64 years 58% male High intensity: 61 years 59% male Paroxysmal or persistent AF | Frequency: Two sessions/week for 12 weeks Intensity: exercise at either low or high intensity (50% (Borg scale 11–13/20) and 80% (Borg scale 16–18/20) of maximal perceived exertion, respectively) Time: 60 min Type: 20 min interval exercising on ergometer bike, 20 min varying circuit exercise on the floor. | Follow-up: 16 weeks. SAE: All hospital admissions: 19 patients in each group. Physical capacity: V˙O2-peak: No difference between groups: Mean diff. -0.76 mL O2/kg/min, 95% CI -3.22 ± 1.70. AF specific outcomes: Burden of AF measured by daily electrocardiography-reporting for 12 weeks. Results: No statistical difference between low and high intensity exercise for both unadjusted (IRR 0.983, 95% CI 0.39–2.46, p = 0.971) and adjusted analyses (IRR 0.742, 95% CI 0.29–1.91, p = 0.538). No serious adverse events in both groups. Three unserious adverse events reported in low intensity group and 5 in high intensity group, including symptoms of arrhythmia, hospital admission, AF ahead of an exercise session, and noncardiac complaints. |
| Wahlström, 2017 | n = 80 Intervention: 64 years 48% male Control: 63 years 72%male Paroxysmal AF | Frequency: One session/week for 12 weeks Intensity: NR (predict light) Time: 30 min Type: Mediyoga (a therapeutic form of yoga evolved from Kundalini yoga). It is calm, meditative yoga based on deep breathing. | Follow-up: 14 weeks QoL: SF-36, Mental component scale improved in the intervention but not control: Intervention group: baseline 42.1 (17.6–53.5) to follow-up 50.6 (24.0–55.2) points vs. control group: baseline 53.0) 14.7–56.0) to follow-up 52.7 (24.5–57.1) points, p = 0.016. Physical component scale no difference within or between groups: Intervention group: 50.2 (27.6–59.1) points vs. control group: 49.0 (29.1–61.6) points, p = 0.837. |
| Joensen, 2019 | n = 52 Intervention: 62 years 61% male Control: 60 years 71% male Paroxysmal or persistent AF | Frequency: 2 sessions/week for 12 weeks Intensity: Moderate-to-vigorous intensity (≥ 70% of maximum exercise capacity or 14–16 on the Borg scale) Time: 60 min Type: Cardiac rehabilitation | Follow-up: 3, 6 and 12-months Physical capacity: Maximum exercise capacity improved in the intervention group from baseline (176 W (SD 48)) to 6 months (190 W (SD 55)). There was no change in the control group. 6MWT was improved in the EG form 613 m (96) to 644 m (84) with no statistically significant differences within or between the groups. AF-QoL-18 significantly improved in the intervention group from 48.4(22.8) to 68.0(15.2) compared with the control group (baseline 51.6 (SD 22.3), 6 months 59.2 (SD 27.3), p = 0.031. There was no statistical difference at 12-months. No statistical difference in AFEQT and EQ-VAS between intervention and control. 20 readmissions for cardiac reasons (mostly AF) in the intervention group and 18 in the control group. 13 direct current cardioversions in the intervention group and 12 in the control group. 7 radiofrequency ablations in the intervention group, and 4 in the control group. |
| Kato, 2019 | n = 68 Intervention: 67 years 71% male Control: 65 years 90% male Persistent AF | Frequency: 1–2 sessions/week, for 24 weeks Intensity: Moderate intensity Time: 60 min Type: Cardiac rehabilitation | Follow-up: 6 months Physical capacity: Significant increases in the 6MWT form 545(123) m to 596 (95) m and also the V˙O2-peak from 17.8 (3.4) mL kg−1 min−1 to 19.8 (4.6) mL kg−1 min−1 in the intervention group after 6 months with no significant changes in the control group. AF specific: During the six-month follow-up period, 21.4% (6 patients) of the intervention group had AF recurrence and 25.8% (8 patients) in the control group with a risk ratio of 0.83 (95%CI, 0.33 to 2.10). 1 thoracic compression fracture not intervention-related, 1 developed hypothyroidism during the intervention period and no cardiovascular adverse events in either group. |
| Melo, 2019 | n = 63 Intervention: 69 years 79% male Control: 66 years 75% male Persistent AF | Frequency: 2 sessions/week, for 6 months Intensity: Vigorous intensity Time: 60 min Type: HIIT | Follow-up: 6 months QoL: HQL-14 improved between the groups but with no significant differences. Physical capacity: Significant increases in V˙O2-peak after 6 months in both intervention and control; from 12.6 (1.7) mL kg−1 min−1 to 15.0 (2.3) mL kg−1 min−1 in intervention and 12.1 (1.7) mL kg−1 min−1 15.6 (2.3) mL kg−1 min−1 in the control. |
| Wahlström, 2020 | n = 152 Intervention 65 years 47% male Active control: 63 years 52% male Control: 64 years 49% male Paroxysmal AF | Frequency: 1 session/week, for 12 weeks Intensity: NR (predict light) Time: 60 min/session Type: Yoga | Follow-up: 12 weeks QoL: SF-36 significantly improved within Medi-yoga intervention group in the Bodily Pain from 70 (27) to 83 (19), (p = 0.014), General Health from 61 (18) to 70 (17), (p = 0.037), Social Function from 75 (28) to 88 (18), (p = 0.029), Mental Health from 64 (16) to 72 (16), (p = 0.030) and Mental Component Summary score from 40 (11) to 46 (9), (p = 0.019), subscales, however, no significance between group effects. No adverse events. |
| Cohort studies | |||
| Ahn, 2021 | n = 66,692 60 years 64% male Newly diagnosed AF | Cohort stratified by: Persistent non-exercisers (30.5%) New exercisers (17.8%), Exercise dropouts (17.4%) Exercise maintainers (34.2%) Exercise “Yes” = performing moderate (>30 min) or vigorous intensity exercise (>20 min), at least once a week. Exercise “No” = not engaging in any moderate or vigorous intensity exercise. Therefore, persistent non-exercisers (No to No), new exercisers (No to Yes), exercise dropouts (Yes to No), and exercise maintainers (Yes to Yes). | Follow-up: 2 years SAE: The new exerciser and exercise maintainer groups were associated with a lower risk of HF compared to the persistent non-exerciser group: the hazard ratios (HRs) (95% Cis) were 0.95 (0.90–0.99) and 0.92 (0.88–0.96), respectively (p < 0.001). Performing exercise any time before or after AF diagnosis was associated with a lower risk of mortality compared to persistent non-exercising: the HR (95% CI) was 0.82 (0.73–0.91) for new exercisers, 0.83 (0.74–0.93) for exercise dropouts, and 0.61 (0.55–0.67) for exercise maintainers (p < 0.001). For ischemic stroke, HRs were 10%–14% lower in patients of the exercise groups, yet differences were statistically insignificant (p = 0.057). Energy expenditure of 1000–1499 MET-min/wk (regular moderate exercise 170–240 min/wk) was consistently associated with a lower risk of each outcome based on a subgroup analysis of the new exerciser group. |
| Bonnesen, 2021 | n = 1410 75 years 46% female Participants had AF risk factors but no prior AF diagnosis | A dynamic parameter describing within-individual changes in daily physical activity, i.e., average daily activity in the last week compared to the previous 100 days, was computed and used to model the onset of AF. | Follow-up: 3.5 years A 1 h decrease in average daily physical activity was associated with AF onset the next day (odds ratio 1.24 (1.18–1.31)). This effect was modified by overall level of activity (p < 0.001 for interaction), and the signal was strongest in the tertile of participants with lowest activity overall (low: 1.62 (1.41–1.86), mid: 1.27 (1.16–1.39), and high: 1.10 (1.01–1.19)). |
| Buckley, 2021a | n = 23,894 68 years 30% female Included paroxysmal, persistent and permanent AF | Comprehensive cardiac rehabilitation programme * | Follow-up: 18 months Exercise-based CR was associated with 68% lower odds of all-cause mortality (odds ratio, 0.32; 95% CI, 0.29–0.35), 44% lower odds of rehospitalisation (0.56; 95% CI, 0.53–0.59), and 16% lower odds of incident stroke (0.84; 95% CI, 0.72–0.99) compared with propensity-score matched controls. The beneficial association of exercise-based CR on all-cause mortality was independent of sex, older age, comorbidities, and AF subtype. |
| Buckley, 2021b | n = 9808 70 years 32% female Included paroxysmal AF | Comprehensive cardiac rehabilitation programme * | Follow-up: 2 years Progression from paroxysmal AF to sustained AF (persistent/permanent) at 2-year follow-up was proportionally lower with 19.3% (n = 617 of 3197 patients) in the exercise-based CR cohort compared to 24.5% (n = 909 of 3716 patients) in the matched controls (OR 0.74, 95% CI: 0.66–0.83). |
| Garnvik, 2020 | n = 1117 Inactive: 73 years 61% male Not meeting: 71 years 67% male Meeting: 69 years 78% male All AF subtypes | Cohort stratified into 3 groups: (1). Inactive, reflecting no PA or less than once a week. (2). Below guideline amount, reflecting < 150 min of moderate intensity or <75 min of vigorous intensity/week. (3). At or above guideline amount, ≥150 min moderate or ≥75 min vigorous intensity/week. | Follow-up: up to 9 years or an event Atrial fibrillation patients meeting PA guidelines had lower risk of all-cause (hazard ratio (HR) 0.55, 95% confidence interval (CI) 0.41–0.75) and CVD mortality (HR 0.54, 95% CI 0.34–0.86) compared with inactive patients. The respective HRs for CVD morbidity and stroke were 0.78 (95% CI 0.58–1.04) and 0.70 (95% CI 0.42–1.15). Each 1-MET higher eCRF was associated with a lower risk of all-cause (HR 0.88, 95% CI 0.81–0.95), CVD mortality (HR 0.85, 95% CI 0.76–0.95), and morbidity (HR 0.88, 95% CI 0.82–0.95). |
| Hegbom, 2006 (Originally an RCT, but results presented as a single arm pre-post study) | n = 30 62 years 88% male Permanent AF | Frequency: 3 sessions/week for 8 weeks Intensity: 70–90% of maximum heart rate Time: 1.25 h Type: Three 15 min periods of aerobics at HRmax, interrupted by strengthening exercise for the back, thighs, and stomach 15 min of stretching and relaxation | Follow-up: 8 weeks Physical capacity: Increase in cumulated work at Borg-17 scale: The intervention group (39% ± 38%) and control group (42% ± 35%). Quality of life change to follow-up, SF-36 (within intervention group): from 49 ± 6 pre-training to 52 ± 6 post-training (p < 0.05). |
| Lakkireddy, 2013 | n = 49 61 years 47% male Paroxysmal AF | Frequency: 2 sessions/week for 12 weeks Intensity: NR (predict light) Time: NR Type: Yoga | Follow-up: 12 weeks AF specific: Yoga training reduced symptomatic AF episodes (3.8 ± 3 vs. 2.1 ± 2.6, p < 0.001), symptomatic non-AF episodes (2.9 ± 3.4 vs. 1.4 ± 2.0; p < 0.001), asymptomatic AF episodes (0.12 ± 0.44 vs. 0.04 ± 0.20; p < 0.001). QoL: SF-36 significantly improved after the intervention period (Physical Functioning from 85.0 (80.0–95.0) to 90.0 (85.0–95.0), (p = 0.017), General Health from 65.0 (50.0–77.5) to 75.0 (65.0–82.5), (p < 0.001), Vitality from 84.0 (68.0–88.0) to 91.0 (80.0–95.8), (p < 0.001), Social Functioning from 100.0 (75.0–100.0) to 100.0 (90.0–100.0), (p = 0.019), and Mental Health from 75.0 (65.0–85.0) to 80.0 (70.0–86.0), (p < 0.001). No adverse events. |
| Younis, 2018 | n = 304 (pre-post AF arm) 68 years 76% male AF subtype NR | Frequency: 2 sessions/week for 6 months Intensity: NR Time: 60 min Type: Cardiac rehabilitation | Follow-up: 6-months Physical capacity: Significant improvement (delta > 5%) was achieved among 194 (64%) patients with AF. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckley, B.J.R.; Risom, S.S.; Boidin, M.; Lip, G.Y.H.; Thijssen, D.H.J. Atrial Fibrillation Specific Exercise Rehabilitation: Are We There Yet? J. Pers. Med. 2022, 12, 610. https://doi.org/10.3390/jpm12040610
Buckley BJR, Risom SS, Boidin M, Lip GYH, Thijssen DHJ. Atrial Fibrillation Specific Exercise Rehabilitation: Are We There Yet? Journal of Personalized Medicine. 2022; 12(4):610. https://doi.org/10.3390/jpm12040610
Chicago/Turabian StyleBuckley, Benjamin J. R., Signe S. Risom, Maxime Boidin, Gregory Y. H. Lip, and Dick H. J. Thijssen. 2022. "Atrial Fibrillation Specific Exercise Rehabilitation: Are We There Yet?" Journal of Personalized Medicine 12, no. 4: 610. https://doi.org/10.3390/jpm12040610
APA StyleBuckley, B. J. R., Risom, S. S., Boidin, M., Lip, G. Y. H., & Thijssen, D. H. J. (2022). Atrial Fibrillation Specific Exercise Rehabilitation: Are We There Yet? Journal of Personalized Medicine, 12(4), 610. https://doi.org/10.3390/jpm12040610