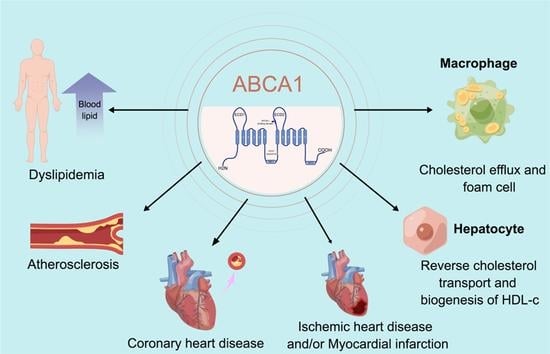
Role of ABCA1 in Cardiovascular Disease
Abstract
:1. Introduction
2. Transcription Regulation of the ABCA1 Gene
3. ABCA1 Gene Mutation and Single Nucleotide Polymorphism
4. Association of Gene Polymorphism with Cardiovascular Risk in Different Ethnicities and Sexes
5. Protective Polymorphism Related to the APOA-I Pathway
6. ABCA1 Protein
6.1. ABCA1 Structure and Distribution
6.2. ABCA1 Post-Translational Modifications
6.2.1. ABCA1 Glycosylation
6.2.2. ABCA1 Ubiquitination
6.2.3. ABCA1 Phosphorylation
6.2.4. ABCA1 Palmitoylation
6.3. Mechanisms of ABCA1 That Regulate Cholesterol Homeostasis
7. ABCA1 and Cardiovascular Diseases
7.1. Dyslipidemia
7.2. Atherosclerosis
7.3. Ischemia/Reperfusion and Ischemic Heart Disease
7.4. Myocardial Infarction
7.5. CHD
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organ). 2017 Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 20 February 2022).
- Jan, S.; Laba, T.L.; Essue, B.M.; Gheorghe, A.; Muhunthan, J.; Engelgau, M.; Mahal, A.; Griffiths, U.; McIntyre, D.; Meng, Q.; et al. Action to address the household economic burden of non-communicable diseases. Lancet 2018, 391, 2047–2058. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- United Nations: World Population Prospects: The 2019 Revision. Available online: https://population.un.org/wpp/ (accessed on 20 February 2022).
- Paneni, F.; Diaz Canestro, C.; Libby, P.; Luscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Redberg, R.F.; Meier, P. Saturated fat does not clog the arteries: Coronary heart disease is a chronic inflammatory condition, the risk of which can be effectively reduced from healthy lifestyle interventions. Br. J. Sports Med. 2017, 51, 1111–1112. [Google Scholar] [CrossRef] [PubMed]
- Rhainds, D.; Tardif, J.C. From HDL-cholesterol to HDL-function: Cholesterol efflux capacity determinants. Curr. Opin. Lipidol. 2019, 30, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Langmann, T. Structure, function and regulation of the ABC1 gene product. Curr. Opin. Lipidol. 2001, 12, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281–290. [Google Scholar] [CrossRef]
- Kozlowska-Rup, D.; Czekaj, P. Barrier role of ABC family of proteins in human placenta. Ginekol. Pol. 2011, 82, 56–63. [Google Scholar]
- Mahringer, A.; Fricker, G. ABC transporters at the blood-brain barrier. Expert Opin. Drug Metab. Toxicol. 2016, 12, 499–508. [Google Scholar] [CrossRef]
- Miller, D.S. Regulation of ABC transporters at the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. ABC transporter regulation by signaling at the blood-brain barrier: Relevance to pharmacology. Adv. Pharmacol. 2014, 71, 1–24. [Google Scholar] [PubMed]
- Hartz, A.M.; Bauer, B. ABC transporters in the CNS—An inventory. Curr. Pharm. Biotechnol. 2011, 12, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Brunham, L.R.; Singaraja, R.R.; Duong, M.; Timmins, J.M.; Fievet, C.; Bissada, N.; Kang, M.H.; Samra, A.; Fruchart, J.C.; McManus, B.; et al. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 548–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Parks, J.S.; Kuipers, F.; Hayden, M.R. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ. Res. 2006, 99, 672–674. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, J.; Ietta, F.; Giacomello, E.; Bechi, N.; Romagnoli, R.; Fava, A.; Paulesu, L. Expression and localization of ATP binding cassette transporter A1 (ABCA1) in first trimester and term human placenta. Placenta 2010, 31, 423–430. [Google Scholar] [CrossRef]
- Kang, M.H.; Zhang, L.H.; Wijesekara, N.; de Haan, W.; Butland, S.; Bhattacharjee, A.; Hayden, M.R. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2724–2732. [Google Scholar] [CrossRef] [Green Version]
- Chai, A.B.; Ammit, A.J.; Gelissen, I.C. Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Respir Res. 2017, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari-Niaki, A.; Ghanbari-Abarghooi, S.; Rahbarizadeh, F.; Zare-Kookandeh, N.; Gholizadeh, M.; Roudbari, F.; Zare-Kookandeh, A. Heart ABCA1 and PPAR- alpha Genes Expression Responses in Male rats: Effects of High Intensity Treadmill Running Training and Aqueous Extraction of Black Crataegus-Pentaegyna. Res. Cardiovasc. Med. 2013, 2, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.D.; Franklin, V.; Marcel, Y.L. In vivo reverse cholesterol transport from macrophages lacking ABCA1 expression is impaired. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1837–1842. [Google Scholar] [CrossRef] [Green Version]
- Stamatikos, A.; Dronadula, N.; Ng, P.; Palmer, D.; Knight, E.; Wacker, B.K.; Tang, C.; Kim, F.; Dichek, D.A. ABCA1 Overexpression in Endothelial Cells In Vitro Enhances ApoAI-Mediated Cholesterol Efflux and Decreases Inflammation. Hum. Gene Ther. 2019, 30, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Albavera, L.; Dominguez-Perez, M.; Medina-Leyte, D.J.; Gonzalez-Garrido, A.; Villarreal-Molina, T. The Role of the ATP-Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021, 22, 1593. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Vitali, C.; Cuchel, M. ABCA1 and Inflammation: From Animal Models to Humans. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1551–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre-Portoles, C.; Feliu, J.; Reglero, G.; Ramirez de Molina, A. ABCA1 overexpression worsens colorectal cancer prognosis by facilitating tumour growth and caveolin-1-dependent invasiveness, and these effects can be ameliorated using the BET inhibitor apabetalone. Mol. Oncol. 2018, 12, 1735–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, G.; Langmann, T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim. Biophys. Acta 2005, 1735, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, M.H.; Song, Y.; Yee, J.; Song, G.; Gwak, H.S. ABCA1 69C>T Polymorphism and the Risk of Type 2 Diabetes Mellitus: A Systematic Review and Updated Meta-Analysis. Front. Endocrinol. 2021, 12, 639524. [Google Scholar] [CrossRef]
- Oram, J.F. The cholesterol mobilizing transporter ABCA1 as a new therapeutic target for cardiovascular disease. Trends Cardiovasc. Med. 2002, 12, 170–175. [Google Scholar] [CrossRef]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; on behalf of the American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Curini, L.; Amedei, A. Cardiovascular Diseases and Pharmacomicrobiomics: A Perspective on Possible Treatment Relevance. Biomedicines 2021, 9, 1338. [Google Scholar] [CrossRef]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Wang, D.; Hiebl, V.; Schachner, D.; Ladurner, A.; Heiss, E.H.; Atanasov, A.G.; Dirsch, V.M. Soraphen A enhances macrophage cholesterol efflux via indirect LXR activation and ABCA1 upregulation. Biochem. Pharmacol. 2020, 177, 114022. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.G.; Horike, H.; Cha, B.Y.; Uhm, K.O.; Yamauchi, R.; Yamaguchi, T.; Hosono, T.; Iida, K.; Woo, J.T.; Michikawa, M. Honokiol increases ABCA1 expression level by activating retinoid X receptor beta. Biol. Pharm. Bull. 2010, 33, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Costet, P.; Lalanne, F.; Gerbod-Giannone, M.C.; Molina, J.R.; Fu, X.; Lund, E.G.; Gudas, L.J.; Tall, A.R. Retinoic acid receptor-mediated induction of ABCA1 in macrophages. Mol. Cell Biol. 2003, 23, 7756–7766. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Beyer, T.P.; Bramlett, K.S.; Yao, S.; Burris, T.P.; Schmidt, R.J.; Eacho, P.I.; Cao, G. Liver X receptor and retinoic X receptor mediated ABCA1 regulation and cholesterol efflux in macrophage cells-messenger RNA measured by branched DNA technology. Mol. Genet. Metab. 2002, 77, 150–158. [Google Scholar] [CrossRef]
- Soumian, S.; Albrecht, C.; Davies, A.H.; Gibbs, R.G. ABCA1 and atherosclerosis. Vasc. Med. 2005, 10, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.H.; Kamanna, V.S.; Ganji, S.H.; Xiong, X.M.; Kashyap, M.L. Niacin increases HDL biogenesis by enhancing DR4-dependent transcription of ABCA1 and lipidation of apolipoprotein A-I in HepG2 cells. J. Lipid Res. 2012, 53, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Haidar, B.; Denis, M.; Krimbou, L.; Marcil, M.; Genest, J., Jr. cAMP induces ABCA1 phosphorylation activity and promotes cholesterol efflux from fibroblasts. J. Lipid Res. 2002, 43, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, N.; Abe-Dohmae, S.; Ayaori, M.; Tanaka, N.; Kusuhara, M.; Ohsuzu, F.; Yokoyama, S. ATP-binding cassette transporter A1 gene transcription is downregulated by activator protein 2alpha. Doxazosin inhibits activator protein 2alpha and increases high-density lipoprotein biogenesis independent of alpha1-adrenoceptor blockade. Circ. Res. 2007, 101, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Liao, H.; Liu, Y.; Lee, T.S.; Zhu, M.; Wang, X.; Stemerman, M.B.; Zhu, Y.; Shyy, J.Y. Sterol-responsive element-binding protein (SREBP) 2 down-regulates ATP-binding cassette transporter A1 in vascular endothelial cells: A novel role of SREBP in regulating cholesterol metabolism. J. Biol. Chem. 2004, 279, 48801–48807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmann, T.; Porsch-Ozcurumez, M.; Heimerl, S.; Probst, M.; Moehle, C.; Taher, M.; Borsukova, H.; Kielar, D.; Kaminski, W.E.; Dittrich-Wengenroth, E.; et al. Identification of sterol-independent regulatory elements in the human ATP-binding cassette transporter A1 promoter: Role of Sp1/3, E-box binding factors, and an oncostatin M-responsive element. J. Biol. Chem. 2002, 277, 14443–14450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigot, V.; Hamon, Y.; Chambenoit, O.; Alibert, M.; Duverger, N.; Chimini, G. Distinct sites on ABCA1 control distinct steps required for cellular release of phospholipids. J. Lipid Res. 2002, 43, 2077–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, H.; Zhao, X.; Cao, P.; Lei, J.; Yan, N.; Gong, X. Structure of the Human Lipid Exporter ABCA1. Cell 2017, 169, 1228–1239.e10. [Google Scholar] [CrossRef] [Green Version]
- Oram, J.F. Tangier disease and ABCA1. Biochim. Biophys. Acta 2000, 1529, 321–330. [Google Scholar] [CrossRef]
- Babashamsi, M.M.; Koukhaloo, S.Z.; Halalkhor, S.; Salimi, A.; Babashamsi, M. ABCA1 and metabolic syndrome; a review of the ABCA1 role in HDL-VLDL production, insulin-glucose homeostasis, inflammation and obesity. Diabetes Metab. Syndr. 2019, 13, 1529–1534. [Google Scholar] [CrossRef]
- Frikke-Schmidt, R. HDL cholesterol and apolipoprotein A-I concentrations and risk of atherosclerotic cardiovascular disease: Human genetics to unravel causality. Atherosclerosis 2020, 299, 53–55. [Google Scholar] [CrossRef]
- Barter, P.; Genest, J. HDL cholesterol and ASCVD risk stratification: A debate. Atherosclerosis 2019, 283, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Kyriakou, T.; Hodgkinson, C.; Pontefract, D.E.; Iyengar, S.; Howell, W.M.; Wong, Y.K.; Eriksson, P.; Ye, S. Genotypic effect of the -565C>T polymorphism in the ABCA1 gene promoter on ABCA1 expression and severity of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Probst, M.C.; Thumann, H.; Aslanidis, C.; Langmann, T.; Buechler, C.; Patsch, W.; Baralle, F.E.; Dallinga-Thie, G.M.; Geisel, J.; Keller, C.; et al. Screening for functional sequence variations and mutations in ABCA1. Atherosclerosis 2004, 175, 269–279. [Google Scholar] [CrossRef]
- Tan, J.H.; Low, P.S.; Tan, Y.S.; Tong, M.C.; Saha, N.; Yang, H.; Heng, C.K. ABCA1 gene polymorphisms and their associations with coronary artery disease and plasma lipids in males from three ethnic populations in Singapore. Hum. Genet. 2003, 113, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, K.Y.; Clee, S.M.; Zwinderman, A.H.; Engert, J.C.; Singaraja, R.; Loubser, O.; James, E.; Roomp, K.; Hudson, T.J.; Jukema, J.W.; et al. ABCA1 regulatory variants influence coronary artery disease independent of effects on plasma lipid levels. Clin. Genet. 2002, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lutucuta, S.; Ballantyne, C.M.; Elghannam, H.; Gotto, A.M., Jr.; Marian, A.J. Novel polymorphisms in promoter region of atp binding cassette transporter gene and plasma lipids, severity, progression, and regression of coronary atherosclerosis and response to therapy. Circ. Res. 2001, 88, 969–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Ji, Y.; Chen, X.; Song, Y.; Huang, S.; Zhou, C.; Huang, C.; Chen, Z.; Zhang, L.; Ge, J. ABCA1 variants rs2230806 (R219K), rs4149313 (M8831I), and rs9282541 (R230C) are associated with susceptibility to coronary heart disease. J. Clin. Lab. Anal. 2019, 33, e22896. [Google Scholar] [CrossRef]
- Villarreal-Molina, T.; Posadas-Romero, C.; Romero-Hidalgo, S.; Antunez-Arguelles, E.; Bautista-Grande, A.; Vargas-Alarcon, G.; Kimura-Hayama, E.; Canizales-Quinteros, S.; Juarez-Rojas, J.G.; Posadas-Sanchez, R.; et al. The ABCA1 gene R230C variant is associated with decreased risk of premature coronary artery disease: The genetics of atherosclerotic disease (GEA) study. PLoS ONE 2012, 7, e49285. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.F.; Li, Z.Q.; Pan, W. Effect of R219K polymorphism of the ABCA1 gene on the lipid-lowering effect of pravastatin in Chinese patients with coronary heart disease. Clin. Exp. Pharmacol. Physiol. 2009, 36, 567–570. [Google Scholar] [CrossRef]
- Andrikovics, H.; Pongracz, E.; Kalina, E.; Szilvasi, A.; Aslanidis, C.; Schmitz, G.; Tordai, A. Decreased frequencies of ABCA1 polymorphisms R219K and V771M in Hungarian patients with cerebrovascular and cardiovascular diseases. Cerebrovasc. Dis. 2006, 21, 254–259. [Google Scholar] [CrossRef]
- Mokuno, J.; Hishida, A.; Morita, E.; Sasakabe, T.; Hattori, Y.; Suma, S.; Okada, R.; Kawai, S.; Naito, M.; Wakai, K. ATP-binding cassette transporter A1 (ABCA1) R219K (G1051A, rs2230806) polymorphism and serum high-density lipoprotein cholesterol levels in a large Japanese population: Cross-sectional data from the Daiko Study. Endocr. J. 2015, 62, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Zhou, R.; Xu, C.; Feng, G.; Zhou, Y. Genetic variation of the ATP-binding cassette transporter A1 and susceptibility to coronary heart disease. Mol. Genet. Metab. 2011, 103, 81–88. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, H.; Qin, X.Y.; Lu, X.Z.; Yang, B.; Chen, M.L. ATP-binding cassette transporter A1 R219K polymorphism and coronary artery disease in Chinese population: A meta-analysis of 5388 participants. Mol. Biol. Rep. 2012, 39, 11031–11039. [Google Scholar] [CrossRef]
- Wang, N.; Xue, X.H.; Lin, Y.; Fang, L.; Murong, S.; Wu, Z.Y. The R219K polymorphism in the ATP-binding cassette transporter 1 gene has a protective effect on atherothrombotic cerebral infarction in Chinese Han ethnic population. Neurobiol. Aging 2010, 31, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Kitjaroentham, A.; Hananantachai, H.; Tungtrongchitr, A.; Pooudong, S.; Tungtrongchitr, R. R219K polymorphism of ATP binding cassette transporter A1 related with low HDL in overweight/obese Thai males. Arch. Med. Res. 2007, 38, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Doosti, M.; Najafi, M.; Reza, J.Z.; Nikzamir, A. The role of ATP-binding-cassette-transporter-A1 (ABCA1) gene polymorphism on coronary artery disease risk. Transl. Res. 2010, 155, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, T.A.; Mohamed, R.H.; Hagrass, H.A. Increased risk of premature coronary artery disease in Egyptians with ABCA1 (R219K), CETP (TaqIB), and LCAT (4886C/T) genes polymorphism. J. Clin. Lipidol. 2014, 8, 381–389. [Google Scholar] [CrossRef]
- Connelly, P.J.; Azizi, Z.; Alipour, P.; Delles, C.; Pilote, L.; Raparelli, V. The Importance of Gender to Understand Sex Differences in Cardiovascular Disease. Can. J. Cardiol. 2021, 37, 699–710. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Dasinger, J.H.; Alexander, B.T. Gender differences in developmental programming of cardiovascular diseases. Clin. Sci. 2016, 130, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Morselli, E.; Santos, R.S.; Criollo, A.; Nelson, M.D.; Palmer, B.F.; Clegg, D.J. The effects of oestrogens and their receptors on cardiometabolic health. Nat. Rev. Endocrinol. 2017, 13, 352–364. [Google Scholar] [CrossRef]
- Jacobsen, B.K.; Nilssen, S.; Heuch, I.; Kvale, G. Does age at natural menopause affect mortality from ischemic heart disease? J. Clin. Epidemiol. 1997, 50, 475–479. [Google Scholar] [CrossRef]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar]
- Qiu, H.; Tian, B.; Resuello, R.G.; Natividad, F.F.; Peppas, A.; Shen, Y.T.; Vatner, D.E.; Vatner, S.F.; Depre, C. Sex-specific regulation of gene expression in the aging monkey aorta. Physiol. Genom. 2007, 29, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolovou, V.; Marvaki, A.; Karakosta, A.; Vasilopoulos, G.; Kalogiani, A.; Mavrogeni, S.; Degiannis, D.; Marvaki, C.; Kolovou, G. Association of gender, ABCA1 gene polymorphisms and lipid profile in Greek young nurses. Lipids Health Dis. 2012, 11, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Lista, J.; Perez-Martinez, P.; Perez-Jimenez, F.; Garcia-Rios, A.; Fuentes, F.; Marin, C.; Gomez-Luna, P.; Camargo, A.; Parnell, L.D.; Ordovas, J.M.; et al. ABCA1 gene variants regulate postprandial lipid metabolism in healthy men. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa-Kobayashi, K.; Yanagi, H.; Yu, Y.; Endo, K.; Arinami, T.; Hamaguchi, H. Associations between serum high-density lipoprotein cholesterol or apolipoprotein AI levels and common genetic variants of the ABCA1 gene in Japanese school-aged children. Metabolism 2004, 53, 182–186. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, H.; Yang, B.; Zhang, S.; Han, S.; Yin, F.; Feng, Y. Correlation Between ABCA1 Gene Polymorphism and aopA-I and HDL-C in Abdominal Aortic Aneurysm. Med. Sci. Monit. 2016, 22, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Tregouet, D.A.; Ricard, S.; Nicaud, V.; Arnould, I.; Soubigou, S.; Rosier, M.; Duverger, N.; Poirier, O.; Mace, S.; Kee, F.; et al. In-depth haplotype analysis of ABCA1 gene polymorphisms in relation to plasma ApoA1 levels and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 775–781. [Google Scholar] [CrossRef]
- Abdel-Razek, O.; Sadananda, S.N.; Li, X.; Cermakova, L.; Frohlich, J.; Brunham, L.R. Increased prevalence of clinical and subclinical atherosclerosis in patients with damaging mutations in ABCA1 or APOA1. J. Clin. Lipidol. 2018, 12, 116–121. [Google Scholar] [CrossRef]
- Bungert, S.; Molday, L.L.; Molday, R.S. Membrane topology of the ATP binding cassette transporter ABCR and its relationship to ABC1 and related ABCA transporters: Identification of N-linked glycosylation sites. J. Biol. Chem. 2001, 276, 23539–23546. [Google Scholar] [CrossRef] [Green Version]
- Kimanius, D.; Forsberg, B.O.; Scheres, S.H.; Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. Elife 2016, 5, e18722. [Google Scholar] [CrossRef]
- Peelman, F.; Labeur, C.; Vanloo, B.; Roosbeek, S.; Devaud, C.; Duverger, N.; Denefle, P.; Rosier, M.; Vandekerckhove, J.; Rosseneu, M. Characterization of the ABCA transporter subfamily: Identification of prokaryotic and eukaryotic members, phylogeny and topology. J. Mol. Biol. 2003, 325, 259–274. [Google Scholar] [CrossRef]
- Vu, L.D.; Gevaert, K.; De Smet, I. Protein Language: Post-Translational Modifications Talking to Each Other. Trends Plant Sci. 2018, 23, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Cesar-Razquin, A.; Snijder, B.; Frappier-Brinton, T.; Isserlin, R.; Gyimesi, G.; Bai, X.; Reithmeier, R.A.; Hepworth, D.; Hediger, M.A.; Edwards, A.M.; et al. A Call for Systematic Research on Solute Carriers. Cell 2015, 162, 478–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UniProt, C. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar]
- Iatan, I.; Bailey, D.; Ruel, I.; Hafiane, A.; Campbell, S.; Krimbou, L.; Genest, J. Membrane microdomains modulate oligomeric ABCA1 function: Impact on apoAI-mediated lipid removal and phosphatidylcholine biosynthesis. J. Lipid Res. 2011, 52, 2043–2055. [Google Scholar] [CrossRef] [Green Version]
- Eichler, J. Protein glycosylation. Curr. Biol. 2019, 29, R229–R231. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, E. Mollusc N-glycosylation: Structures, Functions and Perspectives. Biomolecules 2021, 11, 1820. [Google Scholar] [CrossRef]
- Cacan, R.; Duvet, S.; Kmiecik, D.; Labiau, O.; Mir, A.M.; Verbert, A. ‘Glyco-deglyco’ processes during the synthesis of N-glycoproteins. Biochimie 1998, 80, 59–68. [Google Scholar] [CrossRef]
- Zauner, G.; Kozak, R.P.; Gardner, R.A.; Fernandes, D.L.; Deelder, A.M.; Wuhrer, M. Protein O-glycosylation analysis. Biol. Chem. 2012, 393, 687–708. [Google Scholar] [CrossRef]
- Van den Steen, P.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 151–208. [Google Scholar] [CrossRef]
- Bandini, G.; Albuquerque-Wendt, A.; Hegermann, J.; Samuelson, J.; Routier, F.H. Protein O- and C-Glycosylation pathways in Toxoplasma gondii and Plasmodium falciparum. Parasitology 2019, 146, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Vliegenthart, J.F.; Casset, F. Novel forms of protein glycosylation. Curr. Opin. Struct. Biol. 1998, 8, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.Q.; Zhang, X.; Zou, Y.; Shi, R.; Cao, J.G.; Xu, S.Y.; Deng, L.F.; Zhou, L.; Gong, Y.; Shu, X.; et al. Nonenzymatic Stereoselective S-Glycosylation of Polypeptides and Proteins. J. Am. Chem. Soc. 2021, 143, 11919–11926. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Zhang, H.; Zhao, Z.; Chen, X. Protein S-Glyco-Modification through an Elimination-Addition Mechanism. J. Am. Chem. Soc. 2020, 142, 9382–9388. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 2018, 59, 749–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.R.; Abe-Dohmae, S.; Ohnishi, T.; Aoki, R.; Morinaga, G.; Okuhira, K.; Ikeda, Y.; Kano, F.; Matsuo, M.; Kioka, N.; et al. Effects of mutations of ABCA1 in the first extracellular domain on subcellular trafficking and ATP binding/hydrolysis. J. Biol. Chem. 2003, 278, 8815–8819. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, M.L.; Mendez, A.J.; Moore, K.J.; Andersson, L.P.; Panjeton, H.A.; Freeman, M.W. ATP-binding cassette transporter A1 contains an NH2-terminal signal anchor sequence that translocates the protein’s first hydrophilic domain to the exoplasmic space. J. Biol. Chem. 2001, 276, 15137–15145. [Google Scholar] [CrossRef] [Green Version]
- Jennelle, L.; Hunegnaw, R.; Dubrovsky, L.; Pushkarsky, T.; Fitzgerald, M.L.; Sviridov, D.; Popratiloff, A.; Brichacek, B.; Bukrinsky, M. HIV-1 protein Nef inhibits activity of ATP-binding cassette transporter A1 by targeting endoplasmic reticulum chaperone calnexin. J. Biol. Chem. 2014, 289, 28870–28884. [Google Scholar] [CrossRef] [Green Version]
- Kamada, Y.; Kida, S.; Hirano, K.I.; Yamaguchi, S.; Suzuki, A.; Hashimoto, C.; Kimura, A.; Sato, M.; Fujii, H.; Sobajima, T.; et al. Hepatic aberrant glycosylation by N-acetylglucosaminyltransferase V accelerates HDL assembly. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G859–G868. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, G.; Scheid, M.; Hammerling, U.; Schlesinger, D.H.; Niall, H.D.; Boyse, E.A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc. Natl. Acad. Sci. USA 1975, 72, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Staszczak, M. Fungal Secondary Metabolites as Inhibitors of the Ubiquitin-Proteasome System. Int. J. Mol. Sci. 2021, 22, 13309. [Google Scholar] [CrossRef]
- Escobar-Henriques, M.; Altin, S.; Brave, F.D. Interplay between the Ubiquitin Proteasome System and Mitochondria for Protein Homeostasis. Curr. Issues Mol. Biol. 2020, 35, 35–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gierisch, M.E.; Giovannucci, T.A.; Dantuma, N.P. Reporter-Based Screens for the Ubiquitin/Proteasome System. Front. Chem. 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickles, S.; Vigie, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlossarek, S.; Frey, N.; Carrier, L. Ubiquitin-proteasome system and hereditary cardiomyopathies. J. Mol. Cell Cardiol. 2014, 71, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciechanover, A. Proteolysis: From the lysosome to ubiquitin and the proteasome. Nat. Rev. Mol. Cell Biol. 2005, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Goldberg, A.L. Proteasome inhibitors: Valuable new tools for cell biologists. Trends Cell Biol. 1998, 8, 397–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, H.; Wu, B.; You, S.; Wu, S.; Lu, S.; Wang, P.; Cao, L.; Zhang, N.; Sun, Y. E3 Ubiquitin ligase NEDD4 familyregulatory network in cardiovascular disease. Int. J. Biol. Sci. 2020, 16, 2727–2740. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, Y.; Li, P.F. Mitochondrial Ubiquitin Ligase in Cardiovascular Disorders. Adv. Exp. Med. Biol. 2017, 982, 327–333. [Google Scholar]
- Martins-Marques, T.; Ribeiro-Rodrigues, T.; Pereira, P.; Codogno, P.; Girao, H. Autophagy and ubiquitination in cardiovascular diseases. DNA Cell Biol. 2015, 34, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.C.; Yin, K.; Fu, Y.C.; Zhang, D.W.; Chen, W.J.; Tang, C.K. Posttranscriptional regulation of ATP-binding cassette transporter A1 in lipid metabolism. DNA Cell Biol. 2013, 32, 348–358. [Google Scholar] [CrossRef]
- Mizuno, T.; Hayashi, H.; Kusuhara, H. Cellular Cholesterol Accumulation Facilitates Ubiquitination and Lysosomal Degradation of Cell Surface-Resident ABCA1. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Aleidi, S.M.; Yang, A.; Sharpe, L.J.; Rao, G.; Cochran, B.J.; Rye, K.A.; Kockx, M.; Brown, A.J.; Gelissen, I.C. The E3 ubiquitin ligase, HECTD1, is involved in ABCA1-mediated cholesterol export from macrophages. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, V.; Kim, M.J.; Gelissen, I.C.; Brown, A.J.; Sandoval, C.; Hallab, J.C.; Kockx, M.; Traini, M.; Jessup, W.; Kritharides, L. Cellular cholesterol regulates ubiquitination and degradation of the cholesterol export proteins ABCA1 and ABCG1. J. Biol. Chem. 2014, 289, 7524–7536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, T.; Hayashi, H.; Naoi, S.; Sugiyama, Y. Ubiquitination is associated with lysosomal degradation of cell surface-resident ATP-binding cassette transporter A1 (ABCA1) through the endosomal sorting complex required for transport (ESCRT) pathway. Hepatology 2011, 54, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Katsube, A.; Hayashi, H.; Kusuhara, H. Pim-1L Protects Cell Surface-Resident ABCA1 From Lysosomal Degradation in Hepatocytes and Thereby Regulates Plasma High-Density Lipoprotein Level. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2304–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, Y.; Takada, M.; Maeda, M.; Kioka, N.; Ueda, K. The COP9 signalosome controls ubiquitinylation of ABCA1. Biochem. Biophys. Res. Commun. 2009, 382, 145–148. [Google Scholar] [CrossRef]
- Boro, M.; Govatati, S.; Kumar, R.; Singh, N.K.; Pichavaram, P.; Traylor, J.G., Jr.; Orr, A.W.; Rao, G.N. Thrombin-Par1 signaling axis disrupts COP9 signalosome subunit 3-mediated ABCA1 stabilization in inducing foam cell formation and atherogenesis. Cell Death Differ. 2021, 28, 780–798. [Google Scholar] [CrossRef]
- Editorial expression of concern: “Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation” [Atherosclerosis volume 248, May 2016, pages 149–159]. Atherosclerosis 2021, 330, 127. [CrossRef]
- Ogura, M.; Ayaori, M.; Terao, Y.; Hisada, T.; Iizuka, M.; Takiguchi, S.; Uto-Kondo, H.; Yakushiji, E.; Nakaya, K.; Sasaki, M.; et al. Proteasomal inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression and cholesterol efflux from macrophages in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1980–1987. [Google Scholar] [CrossRef] [Green Version]
- Iborra, R.T.; Machado-Lima, A.; Okuda, L.S.; Pinto, P.R.; Nakandakare, E.R.; Machado, U.F.; Correa-Giannella, M.L.; Pickford, R.; Woods, T.; Brimble, M.A.; et al. AGE-albumin enhances ABCA1 degradation by ubiquitin-proteasome and lysosomal pathways in macrophages. J. Diabetes Complicat. 2018, 32, 1–10. [Google Scholar] [CrossRef]
- Mujawar, Z.; Tamehiro, N.; Grant, A.; Sviridov, D.; Bukrinsky, M.; Fitzgerald, M.L. Mutation of the ATP cassette binding transporter A1 (ABCA1) C-terminus disrupts HIV-1 Nef binding but does not block the Nef enhancement of ABCA1 protein degradation. Biochemistry 2010, 49, 8338–8349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Xu, L.; Chen, W.; Li, X.; Xia, Q.; Zheng, L.; Duan, Q.; Zhang, H.; Zhao, Y. Reduced Annexin A1 Secretion by ABCA1 Causes Retinal Inflammation and Ganglion Cell Apoptosis in a Murine Glaucoma Model. Front. Cell Neurosci. 2018, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.S.; Hou, W.G.; Li, Y.; Liu, D.B.; Hao, G.Z.; Zhang, H.F.; Li, J.C.; Zhao, J.; Zhang, S.; Liang, G.B.; et al. Unexpected requirement for a binding partner of the syntaxin family in phagocytosis by murine testicular Sertoli cells. Cell Death Differ. 2016, 23, 787–800. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, S.; Singh, N.K.; Mani, A.M.; Rao, G.N. Protease-activated receptor 1 inhibits cholesterol efflux and promotes atherogenesis via cullin 3-mediated degradation of the ABCA1 transporter. J. Biol. Chem. 2018, 293, 10574–10589. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Zhao, Z.; Zhao, Y.; Huang, S. Protein arginine phosphorylation in organisms. Int. J. Biol. Macromol. 2021, 171, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Floyd, B.M.; Drew, K.; Marcotte, E.M. Systematic Identification of Protein Phosphorylation-Mediated Interactions. J. Proteome Res. 2021, 20, 1359–1370. [Google Scholar] [CrossRef]
- Loirand, G.; Guilluy, C.; Pacaud, P. Regulation of Rho proteins by phosphorylation in the cardiovascular system. Trends Cardiovasc. Med. 2006, 16, 199–204. [Google Scholar] [CrossRef]
- Colyer, J. Phosphorylation states of phospholamban. Ann. N. Y. Acad. Sci. 1998, 853, 79–91. [Google Scholar] [CrossRef]
- Arakawa, R.; Hayashi, M.; Remaley, A.T.; Brewer, B.H.; Yamauchi, Y.; Yokoyama, S. Phosphorylation and stabilization of ATP binding cassette transporter A1 by synthetic amphiphilic helical peptides. J. Biol. Chem. 2004, 279, 6217–6220. [Google Scholar] [CrossRef] [Green Version]
- See, R.H.; Caday-Malcolm, R.A.; Singaraja, R.R.; Zhou, S.; Silverston, A.; Huber, M.T.; Moran, J.; James, E.R.; Janoo, R.; Savill, J.M.; et al. Protein kinase A site-specific phosphorylation regulates ATP-binding cassette A1 (ABCA1)-mediated phospholipid efflux. J. Biol. Chem. 2002, 277, 41835–41842. [Google Scholar] [CrossRef] [Green Version]
- Martinez, L.O.; Agerholm-Larsen, B.; Wang, N.; Chen, W.; Tall, A.R. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J. Biol. Chem. 2003, 278, 37368–37374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roosbeek, S.; Peelman, F.; Verhee, A.; Labeur, C.; Caster, H.; Lensink, M.F.; Cirulli, C.; Grooten, J.; Cochet, C.; Vandekerckhove, J.; et al. Phosphorylation by protein kinase CK2 modulates the activity of the ATP binding cassette A1 transporter. J. Biol. Chem. 2004, 279, 37779–37788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, Y.; Hayashi, M.; Abe-Dohmae, S.; Yokoyama, S. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J. Biol. Chem. 2003, 278, 47890–47897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Oram, J.F. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J. Lipid Res. 2007, 48, 1062–1068. [Google Scholar] [PubMed] [Green Version]
- Wang, Y.; Oram, J.F. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a phospholipase D2 pathway. J. Biol. Chem. 2005, 280, 35896–35903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.H.; Xiao, J.; Mo, Z.C.; Yin, K.; Jiang, J.; Cui, L.B.; Tan, C.Z.; Tang, Y.L.; Liao, D.F.; Tang, C.K. Contribution of D4-F to ABCA1 expression and cholesterol efflux in THP-1 macrophage-derived foam cells. J. Cardiovasc. Pharmacol. 2010, 56, 309–319. [Google Scholar] [CrossRef]
- Hu, Y.W.; Ma, X.; Li, X.X.; Liu, X.H.; Xiao, J.; Mo, Z.C.; Xiang, J.; Liao, D.F.; Tang, C.K. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis 2009, 204, e35–e43. [Google Scholar] [CrossRef]
- Liang, H.; Wang, Y. Berberine alleviates hepatic lipid accumulation by increasing ABCA1 through the protein kinase C delta pathway. Biochem. Biophys. Res. Commun. 2018, 498, 473–480. [Google Scholar] [CrossRef]
- Tang, M.; Lu, L.; Huang, Z.; Chen, L. Palmitoylation signaling: A novel mechanism of mitochondria dynamics and diverse pathologies. Acta Biochim. Biophys. Sin. 2018, 50, 831–833. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lu, H.; Fang, C.; Xu, J. Palmitoylation as a Signal for Delivery. Adv. Exp. Med. Biol. 2020, 1248, 399–424. [Google Scholar]
- Martin, B.R.; Wang, C.; Adibekian, A.; Tully, S.E.; Cravatt, B.F. Global profiling of dynamic protein palmitoylation. Nat. Methods 2011, 9, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Hao, J.W.; Wang, X.; Guo, H.; Sun, H.H.; Lai, X.Y.; Liu, L.Y.; Zhu, M.; Wang, H.Y.; Li, Y.F.; et al. DHHC4 and DHHC5 Facilitate Fatty Acid Uptake by Palmitoylating and Targeting CD36 to the Plasma Membrane. Cell Rep. 2019, 26, 209–221.e5. [Google Scholar] [CrossRef] [Green Version]
- Singaraja, R.R.; Kang, M.H.; Vaid, K.; Sanders, S.S.; Vilas, G.L.; Arstikaitis, P.; Coutinho, J.; Drisdel, R.C.; El-Husseini Ael, D.; Green, W.N.; et al. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ. Res. 2009, 105, 138–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamehiro, N.; Zhou, S.; Okuhira, K.; Benita, Y.; Brown, C.E.; Zhuang, D.Z.; Latz, E.; Hornemann, T.; von Eckardstein, A.; Xavier, R.J.; et al. SPTLC1 binds ABCA1 to negatively regulate trafficking and cholesterol efflux activity of the transporter. Biochemistry 2008, 47, 6138–6147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, K.; Tomioka, M.; Ueda, K. Function and regulation of ABCA1—Membrane meso-domain organization and reorganization. FEBS J. 2011, 278, 3190–3203. [Google Scholar] [CrossRef]
- Oram, J.F.; Vaughan, A.M. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006, 99, 1031–1043. [Google Scholar] [CrossRef]
- Yu, X.H.; Tang, C.K. ABCA1, ABCG1, and Cholesterol Homeostasis. Adv. Exp. Med. Biol. 2022, 1377, 95–107. [Google Scholar]
- Ishigami, M.; Ogasawara, F.; Nagao, K.; Hashimoto, H.; Kimura, Y.; Kioka, N.; Ueda, K. Temporary sequestration of cholesterol and phosphatidylcholine within extracellular domains of ABCA1 during nascent HDL generation. Sci. Rep. 2018, 8, 6170. [Google Scholar] [CrossRef] [Green Version]
- Nagata, K.O.; Nakada, C.; Kasai, R.S.; Kusumi, A.; Ueda, K. ABCA1 dimer-monomer interconversion during HDL generation revealed by single-molecule imaging. Proc. Natl. Acad. Sci. USA 2013, 110, 5034–5039. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Silver, D.L.; Thiele, C.; Tall, A.R. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J. Biol. Chem. 2001, 276, 23742–23747. [Google Scholar] [CrossRef] [Green Version]
- Fielding, P.E.; Nagao, K.; Hakamata, H.; Chimini, G.; Fielding, C.J. A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry 2000, 39, 14113–14120. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Mei, X.; Herscovitz, H.; Atkinson, D. N-terminal mutation of apoA-I and interaction with ABCA1 reveal mechanisms of nascent HDL biogenesis. J. Lipid Res. 2019, 60, 44–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Sun, Y.; Welch, C.; Gorelik, A.; Leventhal, A.R.; Tabas, I.; Tall, A.R. Preferential ATP-binding cassette transporter A1-mediated cholesterol efflux from late endosomes/lysosomes. J. Biol. Chem. 2001, 276, 43564–43569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Smith, J.D. Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 11358–11363. [Google Scholar] [CrossRef] [Green Version]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef]
- Koseki, M.; Yamashita, S.; Ogura, M.; Ishigaki, Y.; Ono, K.; Tsukamoto, K.; Hori, M.; Matsuki, K.; Yokoyama, S.; Harada-Shiba, M. Current Diagnosis and Management of Tangier Disease. J. Atheroscler. Thromb. 2021, 28, 802–810. [Google Scholar] [CrossRef]
- Smirnov, G.P.; Malyshev, P.P.; Rozhkova, T.A.; Zubareva, M.Y.; Shuvalova, Y.A.; Rebrikov, D.V.; Titov, V.N. The effect of ABCA1 rs2230806 common gene variant on plasma lipid levels in patients with dyslipidemia. Klin. Lab. Diagn. 2018, 63, 410–413. [Google Scholar]
- Liu, M.; Chung, S.; Shelness, G.S.; Parks, J.S. Hepatic ABCA1 deficiency is associated with delayed apolipoprotein B secretory trafficking and augmented VLDL triglyceride secretion. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862 Pt A, 1035–1043. [Google Scholar] [CrossRef]
- Chung, S.; Gebre, A.K.; Seo, J.; Shelness, G.S.; Parks, J.S. A novel role for ABCA1-generated large pre-beta migrating nascent HDL in the regulation of hepatic VLDL triglyceride secretion. J. Lipid Res. 2010, 51, 729–742. [Google Scholar]
- Serfaty-Lacrosniere, C.; Civeira, F.; Lanzberg, A.; Isaia, P.; Berg, J.; Janus, E.D.; Smith, M.P., Jr.; Pritchard, P.H.; Frohlich, J.; Lees, R.S.; et al. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis 1994, 107, 85–98. [Google Scholar] [CrossRef]
- Wu, Y.R.; Shi, X.Y.; Ma, C.Y.; Zhang, Y.; Xu, R.X.; Li, J.J. Liraglutide improves lipid metabolism by enhancing cholesterol efflux associated with ABCA1 and ERK1/2 pathway. Cardiovasc. Diabetol. 2019, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Li, H.; Zhou, H.F.; Liang, Y.; Tong, M.; Chen, L.; Zheng, X.L.; Zhao, G.J. Mangiferin promotes macrophage cholesterol efflux and protects against atherosclerosis by augmenting the expression of ABCA1 and ABCG1. Aging 2019, 11, 10992–11009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mohibi, S.; Vasilatis, D.M.; Chen, M.; Zhang, J.; Chen, X. Ferredoxin reductase and p53 are necessary for lipid homeostasis and tumor suppression through the ABCA1-SREBP pathway. Oncogene 2022, 41, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Liu, P.; Wang, X.; Luo, J.; Zuo, X.; Jiang, X.; Liu, C.; Li, Y.; Li, N.; Chen, M.; et al. SIRT1 activator E1231 protects from experimental atherosclerosis and lowers plasma cholesterol and triglycerides by enhancing ABCA1 expression. Atherosclerosis 2018, 274, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ding, H.; Gong, X.; Liu, Z.; Lin, Y.; Zhang, Z.; Lin, G. Methyl protodioscin increases ABCA1 expression and cholesterol efflux while inhibiting gene expressions for synthesis of cholesterol and triglycerides by suppressing SREBP transcription and microRNA 33a/b levels. Atherosclerosis 2015, 239, 566–570. [Google Scholar] [CrossRef]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia. Pediatr. Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Miname, M.H.; Rocha, V.Z.; Santos, R.D. The Role of RNA-Targeted Therapeutics to Reduce ASCVD Risk: What Have We Learned Recently? Curr. Atheroscler. Rep. 2021, 23, 40. [Google Scholar] [CrossRef]
- Bell, T.A., 3rd; Liu, M.; Donner, A.J.; Lee, R.G.; Mullick, A.E.; Crooke, R.M. Antisense oligonucleotide-mediated inhibition of angiopoietin-like protein 3 increases reverse cholesterol transport in mice. J. Lipid Res. 2021, 62, 100101. [Google Scholar] [CrossRef]
- Tsimikas, S. RNA-targeted therapeutics for lipid disorders. Curr. Opin. Lipidol. 2018, 29, 459–466. [Google Scholar] [CrossRef]
- Wierzbicki, A.S.; Viljoen, A. Anti-sense oligonucleotide therapies for the treatment of hyperlipidaemia. Expert Opin. Biol. Ther. 2016, 16, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, A.; Azizi, F. Genes associated with low serum high-density lipoprotein cholesterol. Arch. Iran Med. 2014, 17, 444–450. [Google Scholar] [PubMed]
- Bugger, H.; Zirlik, A. Anti-inflammatory Strategies in Atherosclerosis. Hamostaseologie 2021, 41, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; De.eanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Kiss, D.; Rader, D. HDL-cholesterol and cardiovascular disease: Rethinking our approach. Curr. Opin. Cardiol. 2015, 30, 536–542. [Google Scholar] [CrossRef]
- Lawn, R.M.; Wade, D.P.; Couse, T.L.; Wilcox, J.N. Localization of human ATP-binding cassette transporter 1 (ABC1) in normal and atherosclerotic tissues. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 378–385. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Ranalletta, M.; Wang, N.; Han, S.; Terasaka, N.; Li, R.; Welch, C.; Tall, A.R. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007, 117, 3900–3908. [Google Scholar] [CrossRef] [Green Version]
- Attie, A.D. ABCA1: At the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem. Sci. 2007, 32, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Oram, J.F. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim. Biophys. Acta 2009, 1791, 563–572. [Google Scholar] [CrossRef]
- Tan, W.H.; Peng, Z.L.; You, T.; Sun, Z.L. CTRP15 promotes macrophage cholesterol efflux and attenuates atherosclerosis by increasing the expression of ABCA1. J. Physiol. Biochem. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, S.; Guo, Z.; Xing, D.; Chen, W. The crosstalk of ABCA1 and ANXA1: A potential mechanism for protection against atherosclerosis. Mol. Med. 2020, 26, 84. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; He, L.H.; Yu, X.H.; Zhao, Z.W.; Wang, G.; Zou, J.; Wen, F.J.; Zhou, L.; Wan, X.J.; Zhang, D.W.; et al. CXCL12 promotes atherosclerosis by downregulating ABCA1 expression via the CXCR4/GSK3beta/beta-catenin(T120)/TCF21 pathway. J. Lipid Res. 2019, 60, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.D.; Yu, X.H.; Chen, L.Y.; Xie, S.L.; Feng, Y.G.; Yang, R.Z.; Zhao, Z.W.; Li, H.; Wang, G.; Tang, C.K. Myocardin suppression increases lipid retention and atherosclerosis via downregulation of ABCA1 in vascular smooth muscle cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158824. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, C.; Han, X.; Jia, X.; Li, Y.; Liu, C.; Li, N.; Liu, L.; Liu, P.; Jiang, X.; et al. E17241 as a Novel ABCA1 (ATP-Binding Cassette Transporter A1) Upregulator Ameliorates Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e284–e298. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Tang, Y.; Yao, P. Potential Mechanisms and Effects of Efferocytosis in Atherosclerosis. Front. Endocrinol. 2020, 11, 585285. [Google Scholar] [CrossRef]
- Chen, W.; Li, L.; Wang, J.; Zhang, R.; Zhang, T.; Wu, Y.; Wang, S.; Xing, D. The ABCA1-efferocytosis axis: A new strategy to protect against atherosclerosis. Clin. Chim. Acta 2021, 518, 1–8. [Google Scholar] [CrossRef]
- Ju, S.; Chang, X.; Wang, J.; Zou, X.; Zhao, Z.; Huang, Z.; Wang, Y.; Yu, B. Sini Decoction Intervention on Atherosclerosis via PPARgamma-LXRalpha-ABCA1 Pathway in Rabbits. Open Life Sci. 2018, 13, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Huang, H.; Li, Y.; Wang, Y.; Zheng, Y.; Liang, J.; Zhang, S.; Liu, M.; Fang, Z. Yin-xing-tong-mai decoction attenuates atherosclerosis via activating PPARgamma-LXRalpha-ABCA1/ABCG1 pathway. Pharmacol. Res. 2021, 169, 105639. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Ren, P.; Yang, Y.; Li, S.; Qin, X.; Zhang, M.; Zhou, M.; Liu, W. Qing-Xue-Xiao-Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-kappaB pathway regulation. Phytomedicine 2021, 93, 153812. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Danbin, W.; Maojuan, G.; Chun, S.; Bin, L.; Lin, Y.; Yingxin, S.; Guanwei, F.; Yefei, C.; Qing, G.; et al. Ethanol extracts of Danlou tablet attenuate atherosclerosis via inhibiting inflammation and promoting lipid effluent. Pharmacol. Res. 2019, 146, 104306. [Google Scholar] [CrossRef]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARgamma, LXRalpha and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar]
- Tang, Y.; Wu, H.; Shao, B.; Wang, Y.; Liu, C.; Guo, M. Celosins inhibit atherosclerosis in ApoE-/- mice and promote autophagy flow. J. Ethnopharmacol. 2018, 215, 74–82. [Google Scholar] [CrossRef]
- Chen, M. Effects of Chinese Herbal Compound “Xuemai Ning” on Rabbit Atherosclerosis Model and Expression of ABCA1. Int. J. Biomed. Sci. 2013, 9, 153–161. [Google Scholar]
- Yang, R.; Yin, D.; Yang, D.; Liu, X.; Zhou, Q.; Pan, Y.; Li, J.; Li, S. Xinnaokang improves cecal microbiota and lipid metabolism to target atherosclerosis. Lett. Appl. Microbiol. 2021, 73, 779–792. [Google Scholar] [CrossRef]
- Li, H.; Bai, L.; Qin, Q.; Feng, B.L.; Zhang, L.; Wei, F.Y.; Yang, X.F. Research progress on anti-atherosclerosis effect and mechanism of flavonoids compounds mediated by macrophages. Zhongguo Zhong Yao Za Zhi 2020, 45, 2827–2834. [Google Scholar]
- Tan, C.; Zhou, L.; Wen, W.; Xiao, N. Curcumin promotes cholesterol efflux by regulating ABCA1 expression through miR-125a-5p/SIRT6 axis in THP-1 macrophage to prevent atherosclerosis. J. Toxicol. Sci. 2021, 46, 209–222. [Google Scholar] [CrossRef]
- Howard, M.C.; Nauser, C.L.; Farrar, C.A.; Sacks, S.H. Complement in ischaemia-reperfusion injury and transplantation. Semin. Immunopathol. 2021, 43, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Sirotkovic-Skerlev, M.; Plestina, S.; Bilic, I.; Kovac, Z. Pathophysiology of ischaemia-reperfusion injury. Lijec Vjesn 2006, 128, 87–95. [Google Scholar] [PubMed]
- Li, Y.Z.; Wu, X.D.; Liu, X.H.; Li, P.F. Mitophagy imbalance in cardiomyocyte ischaemia/reperfusion injury. Acta Physiol. 2019, 225, e13228. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Li, H.; Irwin, M.G. Myocardial ischaemia reperfusion injury: The challenge of translating ischaemic and anaesthetic protection from animal models to humans. Br. J. Anaesth. 2016, 117 (Suppl. 2), ii44–ii62. [Google Scholar] [CrossRef] [Green Version]
- Berge, K.E.; Leren, T.P. Mutations in APOA-I and ABCA1 in Norwegians with low levels of HDL cholesterol. Clin. Chim. Acta 2010, 411, 2019–2023. [Google Scholar] [CrossRef]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Calabresi, L.; Rossoni, G.; Gomaraschi, M.; Sisto, F.; Berti, F.; Franceschini, G. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-alpha content and enhancing prostaglandin release. Circ. Res. 2003, 92, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Frikke-Schmidt, R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis 2010, 208, 305–316. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kastelein, J.J.; Hayden, M.R. ABCA1 gene mutations, HDL cholesterol levels, and risk of ischemic heart disease. JAMA 2008, 300, 1997–1998. [Google Scholar] [CrossRef]
- Frikke-Schmidt, R.; Nordestgaard, B.G.; Schnohr, P.; Steffensen, R.; Tybjaerg-Hansen, A. Mutation in ABCA1 predicted risk of ischemic heart disease in the Copenhagen City Heart Study Population. J. Am. Coll. Cardiol. 2005, 46, 1516–1520. [Google Scholar] [CrossRef] [Green Version]
- Frikke-Schmidt, R.; Nordestgaard, B.G.; Stene, M.C.; Sethi, A.A.; Remaley, A.T.; Schnohr, P.; Grande, P.; Tybjaerg-Hansen, A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008, 299, 2524–2532. [Google Scholar] [CrossRef]
- Linsel-Nitschke, P.; Tall, A.R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 2005, 4, 193–205. [Google Scholar] [CrossRef]
- Vegh, A.; Komori, S.; Szekeres, L.; Parratt, J.R. Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovasc. Res. 1992, 26, 487–495. [Google Scholar] [CrossRef]
- Imaizumi, S.; Miura, S.; Nakamura, K.; Kiya, Y.; Uehara, Y.; Zhang, B.; Matsuo, Y.; Urata, H.; Ideishi, M.; Rye, K.A.; et al. Antiarrhythmogenic effect of reconstituted high-density lipoprotein against ischemia/reperfusion in rats. J. Am. Coll. Cardiol. 2008, 51, 1604–1612. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Shang, J.; Mu, X.D.; Gao, Z.Y. Protective effect of down-regulated microRNA-27a mediating high thoracic epidural block on myocardial ischemia-reperfusion injury in mice through regulating ABCA1 and NF-kappaB signaling pathway. Biomed. Pharmacother. 2019, 112, 108606. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; White, H.D.; on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef]
- Gossage, J.R. Acute myocardial infarction. Reperfusion strategies. Chest 1994, 106, 1851–1866. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Newby, L.K.; Mills, N.L. A Proposal for Modest Revision of the Definition of Type 1 and Type 2 Myocardial Infarction. Circulation 2019, 140, 1773–1775. [Google Scholar] [CrossRef]
- Iatan, I.; Alrasadi, K.; Ruel, I.; Alwaili, K.; Genest, J. Effect of ABCA1 mutations on risk for myocardial infarction. Curr. Atheroscler. Rep. 2008, 10, 413–426. [Google Scholar] [CrossRef]
- Barter, P.J. Cardioprotective effects of high-density lipoproteins: The evidence strengthens. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1305–1306. [Google Scholar] [CrossRef] [Green Version]
- van Capelleveen, J.C.; Kootte, R.S.; Hovingh, G.K.; Bochem, A.E. Myocardial infarction in a 36-year-old man with combined ABCA1 and APOA-1 deficiency. J. Clin. Lipidol. 2015, 9, 396–399. [Google Scholar] [CrossRef]
- Subramaniam, K.; Babu, L.A.; Shah, N. A case of premature and recurrent myocardial infarction associated with ABCA.1 gene mutation. J. Postgrad. Med. 2021, 67, 29–32. [Google Scholar] [CrossRef]
- Pervaiz, M.A.; Gau, G.; Jaffe, A.S.; Saenger, A.K.; Baudhuin, L.; Ellison, J. A Non-classical Presentation of Tangier Disease with Three ABCA1 Mutations. JIMD Rep. 2012, 4, 109–111. [Google Scholar]
- Shioji, K.; Nishioka, J.; Naraba, H.; Kokubo, Y.; Mannami, T.; Inamoto, N.; Kamide, K.; Takiuchi, S.; Yoshii, M.; Miwa, Y.; et al. A promoter variant of the ATP-binding cassette transporter A1 gene alters the HDL cholesterol level in the general Japanese population. J. Hum. Genet. 2004, 49, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Sheidina, A.M.; Pchelina, S.N.; Demidova, D.V.; Rodygina, T.I.; Taraskina, A.E.; Toperverg, O.B.; Berkovich, O.A.; Demina, E.V.; Shvarts, E.I.; Pogoda, T.V.; et al. Allele Frequency Analysis of Four Single Nucleotide Polymorphisms Locating in Promoter and 5′-Untranslated Regions of ABCAI Gene in Young Men—Survivors From Myocardial Infarction. Kardiologiia 2004, 44, 40–45. [Google Scholar]
- Louwe, M.C.; Lammers, B.; Frias, M.A.; Foks, A.C.; de Leeuw, L.R.; Hildebrand, R.B.; Kuiper, J.; Smit, J.W.A.; Van Berkel, T.J.C.; James, R.W.; et al. Abca1 deficiency protects the heart against myocardial infarction-induced injury. Atherosclerosis 2016, 251, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Shalia, K.; Saranath, D.; Shah, V.K. Peripheral Blood Mononuclear Cell ABCA1 Transcripts and Protein Expression in Acute Myocardial Infarction. J. Clin. Lab. Anal. 2015, 29, 242–249. [Google Scholar] [CrossRef]
- Rubic, T.; Trottmann, M.; Lorenz, R.L. Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin. Biochem. Pharmacol. 2004, 67, 411–419. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Baumer, Y.; Mehta, N.N.; Dey, A.K.; Powell-Wiley, T.M.; Boisvert, W.A. Cholesterol crystals and atherosclerosis. Eur. Heart J. 2020, 41, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Tunstall-Pedoe, H.; Smith, W.C. Cholesterol as a risk factor for coronary heart disease. Br. Med. Bull. 1990, 46, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.L.; Fried, L.P.; Barrett-Connor, E. Cholesterol, lipoproteins, and coronary heart disease in women. Clin. Chem. 1988, 34, B60–B70. [Google Scholar]
- Williamson, D.R.; Pharand, C. Statins in the prevention of coronary heart disease. Pharmacotherapy 1998, 18, 242–254. [Google Scholar]
- Pullinger, C.R.; O’Connor, P.M.; Naya-Vigne, J.M.; Kunitake, S.T.; Movsesyan, I.; Frost, P.H.; Malloy, M.J.; Kane, J.P. Levels of Prebeta-1 High-Density Lipoprotein Are a Strong Independent Positive Risk Factor for Coronary Heart Disease and Myocardial Infarction: A Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e018381. [Google Scholar] [CrossRef]
- Kane, J.P.; Malloy, M.J. Prebeta-1 HDL and coronary heart disease. Curr. Opin. Lipidol. 2012, 23, 367–371. [Google Scholar] [CrossRef]
- Guey, L.T.; Pullinger, C.R.; Ishida, B.Y.; O’Connor, P.M.; Zellner, C.; Francone, O.L.; Laramie, J.M.; Naya-Vigne, J.M.; Siradze, K.A.; Deedwania, P.; et al. Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am. J. Cardiol. 2011, 108, 360–366. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Naya-Vigne, J.M.; Duchateau, P.N.; Ishida, B.Y.; Mazur, M.; Schoenhaus, S.A.; Zysow, B.R.; Malloy, M.J.; Kunitake, S.T.; Kane, J.P. Measurement of prebeta-1 HDL in human plasma by an ultrafiltration-isotope dilution technique. Anal. Biochem. 1997, 251, 234–240. [Google Scholar] [CrossRef]
- Asztalos, B.F.; Schaefer, E.J.; Horvath, K.V.; Yamashita, S.; Miller, M.; Franceschini, G.; Calabresi, L. Role of LCAT in HDL remodeling: Investigation of LCAT deficiency states. J. Lipid Res. 2007, 48, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Versmissen, J.; Oosterveer, D.M.; Yazdanpanah, M.; Mulder, M.; Dehghan, A.; Defesche, J.C.; Kastelein, J.J.; Sijbrands, E.J. A frequent variant in the ABCA1 gene is associated with increased coronary heart disease risk and a better response to statin treatment in familial hypercholesterolemia patients. Eur. Heart J. 2011, 32, 469–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sameem, M.; Rani, A.; Arshad, M. Association of rs146292819 Polymorphism in ABCA1 Gene with the Risk of Coronary Artery Disease in Pakistani Population. Biochem. Genet. 2019, 57, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Luo, Z.; Jia, A.; Muhammad, I.; Zeng, W.; Shiganmo, A.; Chen, X.; Song, Y. Effects of ABCA1 gene polymorphisms on risk factors, susceptibility and severity of coronary artery disease. Postgrad. Med. J. 2020, 96, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, K.; Zhou, K.; Wei, Z.; Zeng, Z.; He, L.; Wan, C. Quantitative assessment of the effect of ABCA1 R219K polymorphism on the risk of coronary heart disease. Mol. Biol. Rep. 2012, 39, 1809–1813. [Google Scholar] [CrossRef]
- Fan, S.L.; Li, X.; Chen, S.J.; Qi, G.X. ABCA1 rs4149313 polymorphism and susceptibility to coronary heart disease: A meta-analysis. Ann. Hum. Genet. 2014, 78, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.P.; Chen, L.F.; Dang, A.M.; Li, L.Y.; Fang, Q.; Yan, X.W. Association between the ABCA1-565C/T gene promoter polymorphism and coronary heart disease severity and cholesterol efflux in the Chinese Han population. Genet. Test. Mol. Biomark. 2015, 19, 347–352. [Google Scholar] [CrossRef]
- Wang, Q.G.; Guo, Z.G.; Lai, W.Y.; Zha, Z.; Liu, Y.Y.; Liu, L. Detection of single nucleotide polymorphism of all coding regions in ABCA1 gene in patients with coronary heart disease. Nan Fang Yi Ke Da Xue Xue Bao 2006, 26, 42–45. [Google Scholar]
- Lu, Y.; Liu, Y.; Li, Y.; Zhang, H.; Yu, M.; Kanu, J.S.; Qiao, Y.; Tang, Y.; Zhen, Q.; Cheng, Y. Association of ATP-binding cassette transporter A1 gene polymorphisms with plasma lipid variability and coronary heart disease risk. Int. J. Clin. Exp. Pathol. 2015, 8, 13441–13449. [Google Scholar]
- Miroshnikova, V.V.; Panteleeva, A.A.; Pobozheva, I.A.; Razgildina, N.D.; Polyakova, E.A.; Markov, A.V.; Belyaeva, O.D.; Berkovich, O.A.; Baranova, E.I.; Nazarenko, M.S.; et al. ABCA1 and ABCG1 DNA methylation in epicardial adipose tissue of patients with coronary artery disease. BMC Cardiovasc. Disord. 2021, 21, 566. [Google Scholar] [CrossRef]
- An, F.; Liu, C.; Wang, X.; Li, T.; Fu, H.; Bao, B.; Cong, H.; Zhao, J. Effect of ABCA1 promoter methylation on premature coronary artery disease and its relationship with inflammation. BMC Cardiovasc. Disord. 2021, 21, 78. [Google Scholar] [CrossRef]
- Ghaznavi, H.; Mahmoodi, K.; Soltanpour, M.S. A preliminary study of the association between the ABCA1 gene promoter DNA methylation and coronary artery disease risk. Mol. Biol Res. Commun. 2018, 7, 59–65. [Google Scholar] [PubMed]
- Guay, S.P.; Legare, C.; Houde, A.A.; Mathieu, P.; Bosse, Y.; Bouchard, L. Acetylsalicylic acid, aging and coronary artery disease are associated with ABCA1 DNA methylation in men. Clin. Epigenetics 2014, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.J.; Miller, N.E. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 1975, 1, 16–19. [Google Scholar] [CrossRef]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Wool, G.D.; Reardon, C.A. HDL apolipoprotein-related peptides in the treatment of atherosclerosis and other inflammatory disorders. Curr. Pharm. Des. 2010, 16, 3173–3184. [Google Scholar] [CrossRef] [Green Version]
- Ganjali, S.; Gotto, A.M., Jr.; Ruscica, M.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell Physiol. 2018, 233, 9237–9246. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Y.; Kessler, P.S.; Vaughan, A.M.; Oram, J.F. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J. Biol. Chem. 2009, 284, 32336–32343. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Vaughan, A.M.; Oram, J.F. Janus kinase 2 modulates the apolipoprotein interactions with ABCA1 required for removing cellular cholesterol. J. Biol. Chem. 2004, 279, 7622–7628. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.J.; Yin, K.; Fu, Y.C.; Tang, C.K. The interaction of ApoA-I and ABCA1 triggers signal transduction pathways to mediate efflux of cellular lipids. Mol. Med. 2012, 18, 149–158. [Google Scholar] [CrossRef]
- Mulay, V.; Wood, P.; Rentero, C.; Enrich, C.; Grewal, T. Signal transduction pathways provide opportunities to enhance HDL and apoAI-dependent reverse cholesterol transport. Curr. Pharm. Biotechnol. 2012, 13, 352–364. [Google Scholar] [CrossRef]
- Nofer, J.R.; Feuerborn, R.; Levkau, B.; Sokoll, A.; Seedorf, U.; Assmann, G. Involvement of Cdc42 signaling in apoA-I-induced cholesterol efflux. J. Biol. Chem. 2003, 278, 53055–53062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukamoto, K.; Hirano, K.; Yamashita, S.; Sakai, N.; Ikegami, C.; Zhang, Z.; Matsuura, F.; Hiraoka, H.; Matsuyama, A.; Ishigami, M.; et al. Retarded intracellular lipid transport associated with reduced expression of Cdc42, a member of Rho-GTPases, in human aged skin fibroblasts: A possible function of Cdc42 in mediating intracellular lipid transport. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.K.; Yang, H.; Liu, B. Macrophage Biology in Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e77–e81. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xiao, Q.; Wang, L.; Wang, Y.; Wang, D.; Ding, H. Role of ABCA1 in Cardiovascular Disease. J. Pers. Med. 2022, 12, 1010. https://doi.org/10.3390/jpm12061010
Wang J, Xiao Q, Wang L, Wang Y, Wang D, Ding H. Role of ABCA1 in Cardiovascular Disease. Journal of Personalized Medicine. 2022; 12(6):1010. https://doi.org/10.3390/jpm12061010
Chicago/Turabian StyleWang, Jing, Qianqian Xiao, Luyun Wang, Yan Wang, Daowen Wang, and Hu Ding. 2022. "Role of ABCA1 in Cardiovascular Disease" Journal of Personalized Medicine 12, no. 6: 1010. https://doi.org/10.3390/jpm12061010
APA StyleWang, J., Xiao, Q., Wang, L., Wang, Y., Wang, D., & Ding, H. (2022). Role of ABCA1 in Cardiovascular Disease. Journal of Personalized Medicine, 12(6), 1010. https://doi.org/10.3390/jpm12061010