Determinants of Left Atrial Compliance in the Metabolic Syndrome: Insights from the “Linosa Study”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Data
2.3. Echocardiographic and Carotid Ultrasound Examination
2.4. Statistical Analysis
3. Results
3.1. Metabolic and Echocardiographic Parameters
3.2. Left Atrial Anatomy and Function
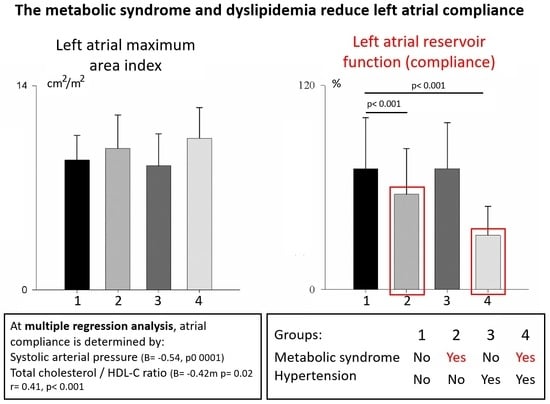
3.3. Subgroup Analysis
3.4. Mortality at 5 Years
4. Discussion
4.1. The Metabolic Syndrome and LV Diastolic Dysfunction
4.2. The Metabolic Syndrome and Left Atrial Remodeling and Function
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, L.; Abhayaratna, W.P. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC. Cardiovasc. Imaging 2017, 10, 65–77. [Google Scholar]
- Abhayaratna, W.P.; Seward, J.B.; Appleton, C.P.; Douglas, P.S.; Oh, J.K.; Tajik, A.J.; Tsang, T.S.M. Left atrial size: Physiologic determinants and clinical applications. J. Am. Coll. Cardiol. 2006, 47, 2357–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, T.S.M.; Barnes, M.E.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am. J. Cardiol. 2002, 90, 1284–1289. [Google Scholar] [CrossRef]
- Benjamin, E.J.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A.; Levy, D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995, 92, 835–841. [Google Scholar] [CrossRef]
- Kizer, J.R.; Bella, J.N.; Palmieri, V.; Liu, J.E.; Best, L.G.; Lee, E.T.; Roman, M.J.; Devereux, R.B. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: The Strong Heart Study (SHS). Am. Heart J. 2006, 151, 412–418. [Google Scholar] [CrossRef]
- Pritchett, A.M.; Jacobsen, S.J.; Mahoney, D.W.; Rodeheffer, R.J.; Bailey, K.R.; Redfield, M.M. Left atrial volume as an index of left atrial size: A population-based study. J. Am. Coll. Cardiol. 2003, 41, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Tsang, T.S.M.; Abhayaratna, W.P.; Barnes, M.E.; Miyasaka, Y.; Gersh, B.J.; Bailey, K.R.; Cha, S.S.; Seward, J.B. Prediction of cardiovascular outcomes with left atrial size: Is volume superior to area or diameter? J. Am. Coll. Cardiol. 2006, 47, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Myreng, Y.; Smiseth, O.A.; Risøe, C. Left ventricular filling at elevated diastolic pressures: Relationship between transmitral Doppler flow velocities and atrial contribution. Am. Heart J. 1990, 119, 620–626. [Google Scholar] [CrossRef]
- Di Salvo, G.; Pacileo, G.; Del Giudice, E.M.; Natale, F.; Limongelli, G.; Verrengia, M.; Rea, A.; Fratta, F.; Castaldi, B.; Gala, S.; et al. Atrial myocardial deformation properties in obese nonhypertensive children. J. Am. Soc. Echocardiogr. 2008, 21, 151–156. [Google Scholar] [CrossRef]
- Kokubu, N.; Yuda, S.; Tsuchihashi, K.; Hashimoto, A.; Nakata, T.; Miura, T.; Ura, N.; Nagao, K.; Tsuzuki, M.; Wakabayashi, C.; et al. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: A possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertens. Res. 2007, 30, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Barbier, P.; Solomon, S.B.; Schiller, N.B.; Glantz, S.A. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999, 100, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suga, H. Importance of atrial compliance in cardiac performance. Circ. Res. 1974, 35, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Chinali, M.; de Simone, G.; Roman, M.J.; Best, L.G.; Lee, E.T.; Russell, M.; Howard, B.V.; Devereux, R.B. Cardiac markers of pre-clinical disease in adolescents with the metabolic syndrome: The strong heart study. J. Am. Coll. Cardiol. 2008, 52, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Simone, G. State of the heart in the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.S.; Cannaday, J.J.; Barlow, C.E.; Mitchell, T.L.; Cooper, K.H.; FitzGerald, S.J. Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am. J. Cardiol. 2008, 102, 689–692. [Google Scholar] [CrossRef]
- Hu, G.; Qiao, Q.; Tuomilehto, J.; Balkau, B.; Borch-Johnsen, K.; Pyorala, K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch. Intern. Med. 2004, 164, 1066–1076. [Google Scholar] [CrossRef]
- Lakka, H.M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef]
- Celentano, A.; Vaccaro, O.; Tammaro, P.; Galderisi, M.; Crivaro, M.; Oliviero, M.; Imperatore, G.; Palmieri, V.; Iovino, V.; Riccardi, G.; et al. Early abnormalities of cardiac function in non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Am. J. Cardiol. 1995, 76, 1173–1176. [Google Scholar] [CrossRef]
- Huang, Y.; Walker, K.E.; Hanley, F.; Narula, J.; Houser, S.R.; Tulenko, T.N. Cardiac systolic and diastolic dysfunction after a cholesterol-rich diet. Circulation 2004, 109, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Van Der Meer, R.W.; Rijzewijk, L.J.; Diamant, M.; Hammer, S.; Schär, M.; Bax, J.J.; Smit, J.W.A.; Romijn, J.A.; De Roos, A.; Lamb, H.J. The ageing male heart: Myocardial triglyceride content as independent predictor of diastolic function. Eur. Heart J. 2008, 29, 1516–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Shin, S.J.; Huang, J.K.; Lin, M.Y.; Lin, Y.H.; Ke, L.Y.; Jiang, H.J.; Tsai, W.C.; Chao, M.F.; Lin, Y.H. The role of postprandial very-low-density lipoprotein in the development of atrial remodeling in metabolic syndrome. Lipids Health Dis. 2020, 19, 1–12. [Google Scholar] [CrossRef]
- Ionin, V.A.; Baranova, E.I.; Zaslavskaya, E.L.; Petrishcheva, E.Y.; Morozov, A.N.; Shlyakhto, E.V. Galectin-3, N-terminal Propeptides of Type I and III Procollagen in Patients with Atrial Fibrillation and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5689. [Google Scholar] [CrossRef]
- Cuspidi, C.; Meani, S.; Fusi, V.; Valerio, C.; Catini, E.; Sala, C.; Sampieri, L.; Magrini, F.; Zanchetti, A. Prevalence and correlates of left atrial enlargement in essential hypertension: Role of ventricular geometry and the metabolic syndrome: The Evaluation of Target Organ Damage in Hypertension study. J. Hypertens. 2005, 23, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Nyman, K.; Granér, M.; Pentikäinen, M.O.; Lundbom, J.; Hakkarainen, A.; Sirén, R.; Nieminen, M.S.; Taskinen, M.R.; Lundbom, N.; Lauerma, K. Metabolic syndrome associates with left atrial dysfunction. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Lucini, D.; Cusumano, G.; Bellia, A.; Kozakova, M.; DiFede, G.; Lauro, R.; Pagani, M. Is reduced baroreflex gain a component of the metabolic syndrome? Insights from the LINOSA study. J. Hypertens. 2006, 24, 361–370. [Google Scholar] [CrossRef]
- Bellia, A.; Giardina, E.; Lauro, D.; Tesauro, M.; Di Fede, G.; Cusumano, G.; Federici, M.; Rini, G.B.; Novelli, G.; Lauro, R.; et al. “The Linosa Study”: Epidemiological and heritability data of the metabolic syndrome in a Caucasian genetic isolate. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 455–461. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Cleeman, J.I. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar]
- Desai, A.N. High Blood Pressure. JAMA 2020, 324, 1254. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, T.; Robinet, S.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Kacharava, G.; Athanassopoulos, G.D.; et al. Echocardiographic reference ranges for normal left atrial function parameters: Results from the EACVI NORRE study. Eur. Hear. J. Cardiovasc. Imaging 2018, 19, 630–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miccoli, R.; Bianchi, C.; Odoguardi, L.; Penno, G.; Caricato, F.; Giovannitti, M.G.; Pucci, L.; Del Prato, S. Prevalence of the metabolic syndrome among Italian adults according to ATP III definition. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; O’Moore-Sullivan, T.; Fang, Z.Y.; Haluska, B.; Leano, R.; Marwick, T.H. Myocardial and vascular dysfunction and exercise capacity in the metabolic syndrome. Am. J. Cardiol. 2005, 96, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Rijzewijk, L.J.; van der Meer, R.W.; Smit, J.W.A.; Diamant, M.; Bax, J.J.; Hammer, S.; Romijn, J.A.; de Roos, A.; Lamb, H.J. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008, 52, 1793–1799. [Google Scholar] [CrossRef] [Green Version]
- Palmiero, P.; Maiello, M.; Passantino, A.; Antoncecchi, E.; Deveredicis, C.; DeFinis, A.; Ostuni, V.; Romano, E.; Mengoli, P.; Caira, D. Correlation between diastolic impairment and lipid metabolism in mild-to-moderate hypertensive postmenopausal women. Am. J. Hypertens. 2002, 15, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Horio, T.; Miyazato, J.; Kamide, K.; Takiuchi, S.; Kawano, Y. Influence of low high-density lipoprotein cholesterol on left ventricular hypertrophy and diastolic function in essential hypertension. Am. J. Hypertens. 2003, 16, 938–944. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.P.; Ting, C.T.; Yang, T.M.; Liu, C.P.; Maughan, W.L.; Chang, M.S.; Kass, D.A. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992, 86, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Schillaci, G.; Vaudo, G.; Reboldi, G.; Verdecchia, P.; Lupattelli, G.; Pasqualini, L.; Porcellati, C.; Mannarino, E. High-density lipoprotein cholesterol and left ventricular hypertrophy in essential hypertension. J. Hypertens. 2001, 19, 2265–2270. [Google Scholar] [CrossRef]
- Khan, A.; Moe, G.W.; Nili, N.; Rezaei, E.; Eskandarian, M.; Butany, J.; Strauss, B.H. The cardiac atria are chambers of active remodeling and dynamic collagen turnover during evolving heart failure. J. Am. Coll. Cardiol. 2004, 43, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Ammar, K.A.; Redfield, M.M.; Mahoney, D.W.; Johnson, M.; Jacobsen, S.J.; Rodeheffer, R.J. Central obesity: Association with left ventricular dysfunction and mortality in the community. Am. Heart J. 2008, 156, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Vasan, R.S. Cardiac function and obesity. Heart 2003, 89, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Valerio, C.; Sala, C.; Negri, F.; Esposito, A.; Masaidi, M.; Giudici, V.; Zanchetti, A.; Mancia, G. Metabolic syndrome and biventricular hypertrophy in essential hypertension. J. Hum. Hypertens. 2009, 23, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clower, B.; Lockwood, W. Light and electron microscopy of diet-induced atrial thrombosis in Swiss mice. II. Recovery on return to a balanced diet. Am. J Pathol. 1972, 66, 65–82. [Google Scholar] [PubMed]
- Gottdiener, J.S.; Reda, D.J.; Williams, D.W.; Materson, B.J. Left atrial size in hypertensive men: Influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J. Am. Coll. Cardiol. 1997, 29, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Rutter, M.K.; Parise, H.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Meigs, J.B.; Nesto, R.W.; Wilson, P.W.; Vasan, R.S. Impact of glucose intolerance and insulin resistance on cardiac structure and function: Sex-related differences in the Framingham Heart Study. Circulation 2003, 107, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskina, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [Green Version]




| All n = 148 | no MetS n = 107 (72%) | MetS n = 41 (28%) | |
|---|---|---|---|
| Age, years | 44.6 ± 16 | 41.3 ± 15 | 54.6 ± 16 ** |
| Gender, M/F | 89/59 | 62/45 | 27/14 |
| BSA, m2 | 1.83 ± 0.22 | 1.81 ± 0.23 | 1.93 ± 0.15 ** |
| BMI, Kg/m2 | 26.3 ± 4.4 | 25.6 ± 4.5 | 28.2 ± 3.6 * |
| Waist/Hip ratio | 0.91 ± 0.01 | 0.89 ± 0.01 | 0.95 ± 0.04 * |
| HR, bpm | 69 ± 11 | 69 ± 11 | 67 ± 9 |
| SAP, mmHg | 130 ± 19 | 125 ± 17 | 145 ± 19 ** |
| DAP, mmHg | 75 ± 9 | 73 ± 9 | 79 ± 9 ** |
| IMT | 0.57 ± 0.09 | 0.54 ± 0.07 | 0.64 ± 0.1 ** |
| HOMA-IR, a.u. | 1.8 ± 1.39 | 1.4 ± 0.65 | 2.6 ± 2.2 ** |
| TC, mg/dL | 203 ± 40 | 194 ± 35 | 228 ± 41 ** |
| HDL-C, mg/dL | 41 ± 11 | 43 ± 11 | 35 ± 8 ** |
| LDL-C, mg/dL | 143 ± 36 | 135 ± 33 | 163 ± 35 ** |
| C/HDL-C ratio | 5.2 ± 1.6 | 4.7 ± 1.2 | 6.8 ± 1.5 ** |
| TG, mg/dL | 114 ± 58 | 89 ± 32 | 185 ± 58 ** |
| CRP, mg/dL | 2.1 ± 2.5 | 2.2 ± 2.6 | 1.8 ± 2.1 |
| Glycemia, mg/dL | 87 ± 13 | 83 ± 9 | 96 ± 17 ** |
| All n = 148 | no MetS n = 107 (72%) | MetS n = 41 (28%) | |
|---|---|---|---|
| Ea, mmHg/mL | 1.85 ± 0.44 | 1.81 ± 0.42 | 2.04 ± 0.44 * |
| LVEDDI, cm/m2 | 2.7 ± 0.3 | 2.7 ± 0.3 | 2.6 ± 0.3 |
| LVFFS, % | 33 ± 6.9 | 33 ± 6.7 | 32 ± 7.4 |
| LVPWd, cm | 0.9 ± 0.1 | 0.91 ± 0.1 | 0.94 ± 0.1 |
| IVSd, cm | 0.9 ± 0.1 | 0.87 ± 0.1 | 0.97 ± 0.1 |
| LVEDHI, cm/m2 | 0.37 ± 0.05 | 0.37 ± 0.05 | 0.39 ± 0.04 |
| LVMI, gr/m2 | 153 ± 36 | 148 ± 32 | 171 ± 44 ** |
| LVESS, 103 dynes/cm2 | 76 ± 22 | 72 ± 19 | 84 ± 25 * |
| LVEDVI, ml/m2 | 59 ± 13 | 59 ± 12 | 61 ± 14 |
| LVEF | 61 ± 6 | 62 ± 6 | 58 ± 7 * |
| LVSVI, mL/m2 | 36 ± 8 | 36 ± 8 | 35 ± 8 |
| Ep, cm/s | 65 ± 17 | 68 ± 14 | 60 ± 21 * |
| Edt, ms | 169 ± 40 | 166 ± 40 | 177 ± 44 |
| Ap, cm/s | 52 ± 16 | 51 ± 15 | 57 ± 20 |
| Ep/Ap | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.2 ± 0.5 * |
| E fractional filling, % | 63 ± 9 | 64 ± 9 | 60 ± 9 * |
| A fractional filling, % | 37 ± 9 | 36 ± 9 | 40 ± 9 * |
| IMT, mm | 0.57 ± 0.09 | 0.54 ± 0.07 | 0.64 ± 0.1 |
| Group 1 no MetS, no HTN n = 90 | Group 2 MetS, no HTN n = 35 | Group 3 no MetS, HTN n = 7 | Group 4 MetS, HTN n = 16 | |
|---|---|---|---|---|
| Age, years | 41 ± 14 | 51 ± 15 * | 42 ± 18 | 77 ± 12 †† |
| Gender, M/F | 44/46 | 29/5 | 5/2 | 11/6 |
| BMI, Kg/m2 | 25.7 ± 4.9 | 27.8 ± 3.3 | 25.8 ± 2.8 | 30 ± 4.9 † |
| Waist/Hip ratio | 0.89 ± 0.08 | 0.95 ± 0.04 ** | 0.91 ± 0.07 | 0.96 ± 0.04 † |
| HR, bpm | 70 ± 11 | 68 ± 9 | 68 ± 12 | 64 ± 7 |
| SAP, mmHg | 124 ± 17 | 145 ± 20 ** | 129 ± 15 | 150 ± 9 † |
| DAP, mmHg | 73 ± 10 | 79 ± 8 * | 74 ± 9 | 75 ± 7 |
| HOMA-IR, a.u. | 1.5 ± 0.7 | 2.8 ± 2.4 ** | 1.4 ± 0.6 | 3 ± 1.2 † |
| TC, mg/dL | 197 ± 35 | 222 ± 36 * | 184 ± 37 | 255 ± 59 †† |
| HDL-C, mg/dL | 44 ± 11 | 34 ± 8 ** | 41 ± 8 | 40 ± 8 |
| LDL-C, mg/dL | 137 ± 33 | 159 ± 32 * | 129 ± 34 | 182 ± 45 † |
| TC/HDL ratio | 4.7 ± 1.2 | 6.9 ± 1.6 ** | 4.6 ± 1.2 | 6.4 ± 0.8 † |
| TG, mg/dL | 91 ± 34 | 188 ± 62 ** | 81 ± 25 | 174 ± 40 †† |
| CRP, mg/dL | 2.1 ± 2.4 | 1.9 ± 2.3 | 3 ± 3.2 | 1.4 ± 0.4 |
| Glycemia, mg/dL | 85 ± 8 | 94 ± 17 ** | 79 ± 9 | 107 ± 12 †† |
| Group 1 No MetS, No HTN n = 90 | Group 2 MetS, No HTN n = 35 | Group 3 No MetS, HTN n = 7 | Group 4 MetS, HTN n = 16 | |
|---|---|---|---|---|
| Ea, mmHg/mL | 1.79 ± 0.44 | 2.06 ± 0.44 * | 1.88 ± 0.37 | 1.91 ± 0.46 |
| LVEDDI, cm/m2 | 2.7 ± 0.3 | 2.6 ± 0.3 | 2.7 ± 0.4 | 2.7 ± 0.3 |
| LVPWd, cm | 0.9 ± 0.1 | 0.95 ± 0.1 | 0.92 ± 0.1 | 0.9 ± 0.1 |
| IVSd, cm | 0.87 ± 0.1 | 0.98 ± 0.1 ** | 0.88 ± 0.1 | 0.93 ± 0.1 |
| LVEDHI | 0.37 ± 0.06 | 0.39 ± 0.04 | 0.37 ± 0.05 | 0.38 ± 0.04 |
| LVMI, g/m2 | 147 ± 33 | 175 ± 47 * | 151 ± 28 | 155 ± 22 |
| LVESS, 103 dynes/cm2 | 72 ± 11 | 82 ± 23 | 75 ± 19 | 97 ± 33 † |
| LVEDVI, mL/m2 | 59 ± 12 | 59 ± 13 | 57 ± 13 | 67 ± 16 |
| LVEF, % | 62 ± 6 | 57 ± 7 * | 63 ± 5 | 61 ± 5 |
| LVSVI, mL/m2 | 36 ± 8 | 34 ± 7 | 36 ± 9 | 41 ± 10 |
| Ep/Ap | 1.4 ± 0.4 | 1.2 ± 0.5 | 1.4 ± 0.5 | 0.8 ± 0.2 † |
| E fractional filling, % | 64 ± 8 | 61 ± 8 | 66 ± 10 | 53 ± 12 † |
| A fractional filling, % | 36 ± 8 | 39 ± 8 | 34 ± 10 | 47 ± 12 † |
| IMT, mm | 0.54 ± 0.07 | 0.64 ± 0.11 ** | 0.55 ± 0.07 | 0.68 ± 0.05 †† |
| Alive n = 128 | CV Deaths n = 4 | All-Cause Deaths n = 20 | |
|---|---|---|---|
| Age, years | 44 ± 16 | 51 ± 13 | 53 ± 15 ** |
| Gender, M/F | 79/49 | 3/1 | 10/10 |
| BMI, Kg/m2 | 26.3 ± 4.5 | 27.4 ± 2.2 | 27.8 ± 4.4 |
| HR, bpm | 69.1 ± 10.7 | 64.3 ± 6.8 | 69.4 ± 10.2 |
| SAP, mmHg | 129.6 ± 19.3 | 135.3 ± 19.8 | 136.5 ± 20.5 |
| DAP, mmHg | 74.6 ± 9.3 | 74.5 ± 9.0 | 76.6 ± 8.5 |
| HOMA-IR, a.u. | 1.8 ± 1.4 | 2.2 ± 0.5 | 2.1 ± 1.2 |
| TC, mg/dL | 202.2 ± 39.8 | 221.7 ± 48.1 | 206.6 ± 42.3 |
| HDL-C, mg/dL | 41.1 ± 10.7 | 32.0 ± 3.5 * | 35.2 ± 8.8 * |
| LDL-C, mg/dL | 142.3 ± 35.8 | 159.7 ± 42.1 | 152.0 ± 39.7 |
| TG, mg/dL | 112.4 ± 57.3 | 175.7 ± 73.7 | 135.4 ± 55.7 |
| Glycemia, mg/dL | 87.0 ± 12.8 | 85.5 ± 7.1 | 91.0 ± 10.2 * |
| LVPWd, cm | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| IVSd, cm | 0.9 ± 0.1 | 1.0 ± 0.1 * | 1.0 ± 0.1 * |
| LVMI, gr/m2 | 151.9 ± 34.3 | 206.2 ± 66.9 * | 169.2 ± 46.3 |
| LVESS, 103 dynes/cm2 | 75.9 ± 21.6 | 77.7 ± 27.8 | 73.3 ± 18.2 |
| LVEDVi, ml/m2 | 58.8 ± 12.0 | 79.3 ± 16.6 * | 63.8 ± 13.2 |
| LVEF, % | 61.0 ± 6.3 | 51.0 ± 7.1 * | 59.3 ± 7.2 |
| LVSVI, ml/m2 | 35.8 ± 8.3 | 39.7 ± 3.4 | 37.2 ± 4.9 |
| Ep/Ap | 1.3 ± 0.4 | 1.8 ± 0.7 | 1.3 ± 0.5 |
| E fractional filling, % | 62.9 ± 9.1 | 69.4 ± 11.6 | 62.2 ± 8.6 |
| A fractional filling, % | 37.1 ± 9.1 | 30.6 ± 11.5 | 37.8 ± 8.6 |
| LAres, % | 68.3 ± 30.2 | 25.0 ± 17.0 * | 55.6 ± 37.3 * |
| LAmaxi, cm2/m2 | 9.1 ± 2.0 | 10.5 ± 2.3 | 9.8 ± 2.2 |
| IMT, mm | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbier, P.; Palazzo Adriano, E.; Lucini, D.; Pagani, M.; Cusumano, G.; De Maria, B.; Dalla Vecchia, L.A. Determinants of Left Atrial Compliance in the Metabolic Syndrome: Insights from the “Linosa Study”. J. Pers. Med. 2022, 12, 1044. https://doi.org/10.3390/jpm12071044
Barbier P, Palazzo Adriano E, Lucini D, Pagani M, Cusumano G, De Maria B, Dalla Vecchia LA. Determinants of Left Atrial Compliance in the Metabolic Syndrome: Insights from the “Linosa Study”. Journal of Personalized Medicine. 2022; 12(7):1044. https://doi.org/10.3390/jpm12071044
Chicago/Turabian StyleBarbier, Paolo, Edvige Palazzo Adriano, Daniela Lucini, Massimo Pagani, Gaspare Cusumano, Beatrice De Maria, and Laura Adelaide Dalla Vecchia. 2022. "Determinants of Left Atrial Compliance in the Metabolic Syndrome: Insights from the “Linosa Study”" Journal of Personalized Medicine 12, no. 7: 1044. https://doi.org/10.3390/jpm12071044
APA StyleBarbier, P., Palazzo Adriano, E., Lucini, D., Pagani, M., Cusumano, G., De Maria, B., & Dalla Vecchia, L. A. (2022). Determinants of Left Atrial Compliance in the Metabolic Syndrome: Insights from the “Linosa Study”. Journal of Personalized Medicine, 12(7), 1044. https://doi.org/10.3390/jpm12071044