Increased CD16a (FcγRIIIA) Expression in The Tumor Microenvironment of Atypical Neurofibromatous Neoplasms of Uncertain Biologic Potential May Be Associated with Progression from Neurofibromas to Atypical Neurofibromas
Abstract
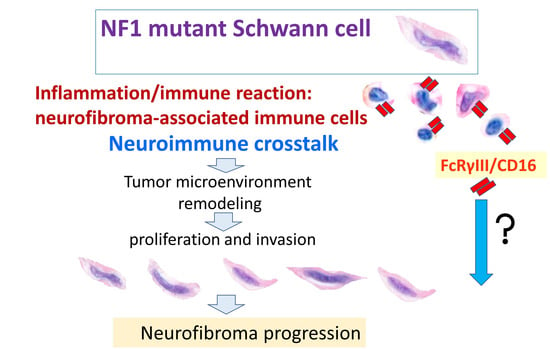
:1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Immunohistochemical Staining and Analysis
2.3. Statistical Analysis
3. Results
3.1. CD16a and CD16b Expression Levels Were Significantly Elevated in ANNUBPs
3.2. TREM2 Expression Was Significantly Increased along with CD16b, Galectin-3 and SOX10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldblum, J.P.; Folpe, A.L.; Weiss, S.W. Enzinger and Weiss’s Soft Tissue Tumors; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Brosseau, J.P.; Liao, C.P.; Le, L.Q. Translating current basic research into future therapies for neurofibromatosis type 1. Br. J. Cancer 2020, 123, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.M.; Antonescu, C.R.; Fletcher, C.D.M.; Kim, A.; Lazar, A.J.; Quezado, M.M.; Reilly, K.M.; Stemmer-Rachamimov, A.; Stewart, D.R.; Viskochil, D.; et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum. Pathol. 2017, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, J.P.; Pichard, D.C.; Legius, E.H.; Wolkenstein, P.; Lavker, R.M.; Blakeley, J.O.; Riccardi, V.M.; Verma, S.K.; Brownell, I.; Le, L.Q. The biology of cutaneous neurofibromas: Consensus recommendations for setting research priorities. Neurology 2018, 91, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Monk, K.R.; Wu, J.; Williams, J.P.; Finney, B.A.; Fitzgerald, M.E.; Filippi, M.D.; Ratner, N. Mast cells can contribute to axon-glial dissociation and fibrosis in peripheral nerve. Neuron Glia Biol. 2007, 3, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.P.; Booker, R.C.; Brosseau, J.P.; Chen, Z.; Mo, J.; Tchegnon, E.; Wang, Y.; Clapp, D.W.; Le, L.Q. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J. Clin. Investig. 2018, 128, 2848–2861. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Prada, C.E.; Jousma, E.; Rizvi, T.A.; Wu, J.; Dunn, R.S.; Mayes, D.A.; Cancelas, J.A.; Dombi, E.; Kim, M.O.; West, B.L.; et al. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 2013, 125, 159–168. [Google Scholar] [CrossRef]
- Choi, K.; Komurov, K.; Fletcher, J.S.; Jousma, E.; Cancelas, J.A.; Wu, J.; Ratner, N. An inflammatory gene signature distinguishes neurofibroma Schwann cells and macrophages from cells in the normal peripheral nervous system. Sci. Rep. 2017, 7, 43315. [Google Scholar] [CrossRef]
- Manich, G.; Gomez-Lopez, A.R.; Almolda, B.; Villacampa, N.; Recasens, M.; Shrivastava, K.; Gonzalez, B.; Castellano, B. Differential Roles of TREM2+ Microglia in Anterograde and Retrograde Axonal Injury Models. Front. Cell. Neurosci. 2020, 14, 567404. [Google Scholar] [CrossRef]
- Yeo, M.K.; Kim, S.; Yoo, H.J.; Suh, K.S.; Kim, K.H. HER2 Expression in Peritoneal Dissemination of High-Grade Serous Ovarian Carcinoma: A Comparative Study of Immunohistochemical Reactivity Using Four HER2 Antibodies. J. Clin. Med. 2022, 11, 6963. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Higham, C.S.; Dombi, E.; Rogiers, A.; Bhaumik, S.; Pans, S.; Connor, S.E.J.; Miettinen, M.; Sciot, R.; Tirabosco, R.; Brems, H.; et al. The characteristics of 76 atypical neurofibromas as precursors to neurofibromatosis 1 associated malignant peripheral nerve sheath tumors. Neuro Oncol. 2018, 20, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.N.; Dombi, E.; Rosenblum, J.S.; Miettinen, M.M.; Lehky, T.J.; Whitcomb, P.O.; Hayes, C.; Scott, G.; Benzo, S.; Widemann, B.C.; et al. Safe marginal resection of atypical neurofibromas in neurofibromatosis type 1. J. Neurosurg. 2019, 133, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Beert, E.; Brems, H.; Daniels, B.; De Wever, I.; Van Calenbergh, F.; Schoenaers, J.; Debiec-Rychter, M.; Gevaert, O.; De Raedt, T.; Van Den Bruel, A.; et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer 2011, 50, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, S.D.; He, Y.; Smith, A.; Jiang, L.; Lu, Q.; Mund, J.; Li, X.; Bessler, W.; Qian, S.; Dyer, W.; et al. Cdkn2a (Arf) loss drives NF1-associated atypical neurofibroma and malignant transformation. Hum. Mol. Genet. 2019, 28, 2752–2762. [Google Scholar] [CrossRef]
- Ge, L.L.; Xing, M.Y.; Zhang, H.B.; Wang, Z.C. Neurofibroma Development in Neurofibromatosis Type 1: Insights from Cellular Origin and Schwann Cell Lineage Development. Cancers 2022, 14, 4513. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, J.; Brosseau, J.P.; Shipman, T.; Wang, Y.; Liao, C.P.; Cooper, J.M.; Allaway, R.J.; Gosline, S.J.C.; Guinney, J.; et al. Spatiotemporal Loss of NF1 in Schwann Cell Lineage Leads to Different Types of Cutaneous Neurofibroma Susceptible to Modification by the Hippo Pathway. Cancer Discov. 2019, 9, 114–129. [Google Scholar] [CrossRef]
- Radomska, K.J.; Coulpier, F.; Gresset, A.; Schmitt, A.; Debbiche, A.; Lemoine, S.; Wolkenstein, P.; Vallat, J.M.; Charnay, P.; Topilko, P. Cellular Origin, Tumor Progression, and Pathogenic Mechanisms of Cutaneous Neurofibromas Revealed by Mice with Nf1 Knockout in Boundary Cap Cells. Cancer Discov. 2019, 9, 130–147. [Google Scholar] [CrossRef]
- Mo, J.; Anastasaki, C.; Chen, Z.; Shipman, T.; Papke, J.; Yin, K.; Gutmann, D.H.; Le, L.Q. Humanized neurofibroma model from induced pluripotent stem cells delineates tumor pathogenesis and developmental origins. J. Clin. Investig. 2021, 131, e139807. [Google Scholar] [CrossRef]
- Jiang, C.; McKay, R.M.; Le, L.Q. Tumorigenesis in neurofibromatosis type 1: Role of the microenvironment. Oncogene 2021, 40, 5781–5787. [Google Scholar] [CrossRef]
- Chenoweth, A.M.; Wines, B.D.; Anania, J.C.; Mark Hogarth, P. Harnessing the immune system via FcgammaR function in immune therapy: A pathway to next-gen mAbs. Immunol. Cell Biol. 2020, 98, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Barb, A.W. Fc gamma receptor compositional heterogeneity: Considerations for immunotherapy development. J. Biol. Chem. 2021, 296, 100057. [Google Scholar] [CrossRef] [PubMed]
- Victor, A.R.; Weigel, C.; Scoville, S.D.; Chan, W.K.; Chatman, K.; Nemer, M.M.; Mao, C.; Young, K.A.; Zhang, J.; Yu, J.; et al. Epigenetic and Posttranscriptional Regulation of CD16 Expression during Human NK Cell Development. J. Immunol. 2018, 200, 565–572. [Google Scholar] [CrossRef]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.; Quek, J.K.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef] [PubMed]
- Wolbert, J.; Li, X.; Heming, M.; Mausberg, A.K.; Akkermann, D.; Frydrychowicz, C.; Fledrich, R.; Groeneweg, L.; Schulz, C.; Stettner, M.; et al. Redefining the heterogeneity of peripheral nerve cells in health and autoimmunity. Proc. Natl. Acad. Sci. USA 2020, 117, 9466–9476. [Google Scholar] [CrossRef]
- Ding, P.; Wang, W.; Wang, J.; Yang, Z.; Xue, L. Expression of tumor-associated macrophage in progression of human glioma. Cell Biochem. Biophys. 2014, 70, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Wacker, K.; Ringelstein, E.B.; Hickey, W.F.; Imai, Y.; Kiefer, R. Rapid response of identified resident endoneurial macrophages to nerve injury. Am. J. Pathol. 2001, 159, 2187–2197. [Google Scholar] [CrossRef]
- Perego, C.; Fumagalli, S.; Zanier, E.R.; Carlino, E.; Panini, N.; Erba, E.; De Simoni, M.G. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiol. Dis. 2016, 96, 284–293. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Springer, M.G.; Choi, K.; Jousma, E.; Rizvi, T.A.; Dombi, E.; Kim, M.O.; Wu, J.; Ratner, N. STAT3 inhibition reduces macrophage number and tumor growth in neurofibroma. Oncogene 2019, 38, 2876–2884. [Google Scholar] [CrossRef]
- Jay, T.R.; von Saucken, V.E.; Landreth, G.E. TREM2 in Neurodegenerative Diseases. Mol. Neurodegener. 2017, 12, 56. [Google Scholar] [CrossRef]
- De Picker, L.J.; Victoriano, G.M.; Richards, R.; Gorvett, A.J.; Lyons, S.; Buckland, G.R.; Tofani, T.; Norman, J.L.; Chatelet, D.S.; Nicoll, J.A.R.; et al. Immune environment of the brain in schizophrenia and during the psychotic episode: A human post-mortem study. Brain Behav. Immun. 2021, 97, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Tzekova, N.; Heinen, A.; Kury, P. Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. J. Clin. Immunol. 2014, 34 (Suppl. 1), S86–S104. [Google Scholar] [CrossRef] [PubMed]



| Case | Neurofibroma (NF) | ANNUBP | MPNST | p |
|---|---|---|---|---|
| No. (total: 38) | 18 | 14 | 6 | |
| Age (mean) | 54.50 | 56.43 | 47.33 | 0.613 * |
| Sex (F/M) | 8/10 | 9/5 | 0/6 | 0.265 ** |
| Type (diffuse/plexiform) | 11/7 | 9/5 | 1/5 | 0.854 ** |
| Variable (Mean) | No. | CD16a | CD16b | CD68 | Galectin-3 | SOX10 | TREM2 |
|---|---|---|---|---|---|---|---|
| Age (rho) | 38 | 0.319 | 0.233 | 0.173 | −0.101 | 0.365 * | 0.256 |
| Gender (M-W) | 38 | p = 0.014 | p = 0.078 | p = 0.121 | p = 0.045 | p = 0.294 | p = 0.399 |
| Female | 17 | 63.92 | 56.03 | 28.90 | 84.80 | 111.24 | 76.97 |
| Male | 21 | 23.64 | 28.61 | 18.46 | 57.81 | 90.65 | 62.15 |
| Diagnosis (K-W) | 38 | p < 0.001 | p < 0.001 | p = 0.001 | p = 0.002 | p = 0.011 | p = 0.263 |
| NF | 18 | 15.51 | 10.00 | 18.81 | 69.07 | 81.58 | 60.13 |
| ANNUBP | 14 | 91.37 | 94.33 | 36.62 | 94.37 | 150.70 | 84.95 |
| MPNST | 6 | 4.11 | 8.77 | 4.61 | 15.17 | 36.09 | 56.96 |
| Type (M-W) | 38 | p = 0.246 | p = 0.736 | p = 0.826 | p = 0.018 | p = 0.051 | p = 0.311 |
| diffuse | 21 | 52.25 | 46.24 | 23.86 | 85.88 | 119.43 | 76.50 |
| Plexiform | 17 | 28.57 | 34.25 | 22.24 | 50.12 | 75.69 | 59.23 |
| Disease-Free Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| HR | Lower | Upper | p Value | HR | Lower | Upper | p Value | |
| CD16a | 0.989 | 0.968 | 1.011 | 0.333 | 1.101 | 0.671 | 1.807 | 0.702 |
| CD16b | 1.014 | 0.991 | 1.037 | 0.243 | 0.907 | 0.571 | 1.440 | 0.678 |
| CD68 | 0.996 | 0.966 | 1.027 | 0.787 | 1.056 | 0.796 | 1.400 | 0.708 |
| Galectin3 | 1.003 | 0.991 | 1.016 | 0.608 | 0.928 | 0.621 | 1.386 | 0.714 |
| SOX10 | 0.993 | 0.986 | 1.001 | 0.085 | 0.976 | 0.891 | 1.068 | 0.596 |
| TREM2 | 1.002 | 0.985 | 1.018 | 0.834 | 1.174 | 0.904 | 1.525 | 0.229 |
| Spearman’s Rho | CD16a | CD16b | CD68 | Galectin3 | TREM2 | SOX10 | |
|---|---|---|---|---|---|---|---|
| CD16a | Correlation coefficient | 1.000 | 0.830 ** | 0.612 ** | 0.349 * | 0.245 | 0.402 * |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.032 | 0.138 | 0.012 | ||
| No. | 38 | 38 | 38 | 38 | 38 | 38 | |
| CD16b | Correlation coefficient | 0.830 ** | 10.000 | 0.733 ** | 0.328 * | 0.347 * | 0.337 * |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.044 | 0.033 | 0.038 | ||
| No. | 38 | 38 | 38 | 38 | 38 | 38 | |
| CD68 | Correlation coefficient | 0.612 ** | 0.733 ** | 1.000 | 0.382 * | 0.306 | 0.373 * |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.018 | 0.062 | 0.021 | ||
| No. | 38 | 38 | 38 | 38 | 38 | 38 | |
| Galectin3 | Correlation coefficient | 0.349 * | 0.328 * | 0.382 * | 1.000 | 0.468 ** | 0.257 |
| Sig. (2-tailed) | 0.032 | 0.044 | 0.018 | 0.003 | 0.119 | ||
| No. | 38 | 38 | 38 | 38 | 38 | 38 | |
| TREM2 | Correlation coefficient | 0.245 | 0.347 * | 0.306 | 0.468 ** | 1.000 | 0.354 * |
| Sig. (2-tailed) | 0.138 | 0.033 | 0.062 | 0.003 | 0.029 | ||
| No. | 38 | 38 | 38 | 38 | 38 | 38 | |
| SOX10 | Correlation coefficient | 0.402 * | 0.337 * | 0.373 * | 0.257 | 0.354 * | 1.000 |
| Sig. (2-tailed) | 0.012 | 0.038 | 0.021 | 0.119 | 0.029 | ||
| No. | 38 | 38 | 38 | 38 | 38 | 38 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, M.-K.; Koh, Y.J.; Park, J.-I.; Kim, K.-H. Increased CD16a (FcγRIIIA) Expression in The Tumor Microenvironment of Atypical Neurofibromatous Neoplasms of Uncertain Biologic Potential May Be Associated with Progression from Neurofibromas to Atypical Neurofibromas. J. Pers. Med. 2023, 13, 1720. https://doi.org/10.3390/jpm13121720
Yeo M-K, Koh YJ, Park J-I, Kim K-H. Increased CD16a (FcγRIIIA) Expression in The Tumor Microenvironment of Atypical Neurofibromatous Neoplasms of Uncertain Biologic Potential May Be Associated with Progression from Neurofibromas to Atypical Neurofibromas. Journal of Personalized Medicine. 2023; 13(12):1720. https://doi.org/10.3390/jpm13121720
Chicago/Turabian StyleYeo, Min-Kyung, Yeong Jun Koh, Jong-Il Park, and Kyung-Hee Kim. 2023. "Increased CD16a (FcγRIIIA) Expression in The Tumor Microenvironment of Atypical Neurofibromatous Neoplasms of Uncertain Biologic Potential May Be Associated with Progression from Neurofibromas to Atypical Neurofibromas" Journal of Personalized Medicine 13, no. 12: 1720. https://doi.org/10.3390/jpm13121720
APA StyleYeo, M. -K., Koh, Y. J., Park, J. -I., & Kim, K. -H. (2023). Increased CD16a (FcγRIIIA) Expression in The Tumor Microenvironment of Atypical Neurofibromatous Neoplasms of Uncertain Biologic Potential May Be Associated with Progression from Neurofibromas to Atypical Neurofibromas. Journal of Personalized Medicine, 13(12), 1720. https://doi.org/10.3390/jpm13121720