Urine Immunoglobin G Greater Than 2.45 mg/L Has a Correlation with the Onset and Progression of Diabetic Kidney Disease: A Retrospective Cohort Study
Abstract
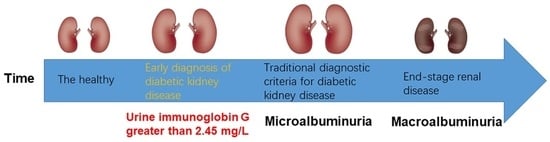
:1. Introduction
2. Methods
2.1. Study Objects
2.2. Definition of Onset and Progression of DKD
2.2.1. Definition of the Onset of DKD
2.2.2. Definition of the Progression of DKD
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Features of Samples
3.2. Rates of the Onset and Progression of DKD between Groups
3.3. The Relationship between Urine IgG Greater Than 2.45 mg/L and the Onset and Progression of DKD
3.4. Kaplan–Meier Curves for DKD Onset and Progression
3.5. Receiver Operating Characteristic Curves for DKD Onset and Progression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Long, J.; Jiang, W.; Shi, Y.; He, X.; Zhou, Z.; Li, Y.; Yeung, R.O.; Wang, J.; Matsushita, K.; et al. Trends in Chronic Kidney Disease in China. N. Engl. J. Med. 2016, 375, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Breyer, J.A. Diabetic Nephropathy in Insulin-Dependent Patients. Am. J. Kidney Dis. 1992, 20, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Gurley, S.B.; Ghosh, S.; Johnson, S.A.; Azushima, K.; Sakban, R.B.; George, S.E.; Maeda, M.; Meyer, T.W.; Coffman, T.M. Inflammation and Immunity Pathways Regulate Genetic Susceptibility to Diabetic Nephropathy. Diabetes 2018, 67, 2096–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulou-Marketou, N.; Kanaka-Gantenbein, C.; Marketos, N.; Chrousos, G.P.; Papassotiriou, I. Biomarkers of diabetic nephropathy: A 2017 update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, S.; Shen, X.; Zhang, S.; Wang, J.; Zuo, M.; Cui, X.; Gao, Z.; Yang, J.; Zhu, H.; et al. Evaluation of urinary biomarkers for prediction of diabetic kidney disease: A propensity score matching analysis. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819891110. [Google Scholar] [CrossRef]
- Berg, U.B.; Bohman, S.O.; Widstam-Attorps, U. Renal histological changes in relation to renal function and urinary protein excretion in IgA nephropathy. Arch. Dis. Child. 1991, 66, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Djukanović, L.; Djordjević, V.; Ležaić, V.; Čukuranović, R.; Marić, I.; Bukvić, D.; Marinković, J.; Čukuranović, J.; Rajić, M.; Stefanović, V. Urinary protein patterns in patients with Balkan endemic nephropathy. Int. Urol. Nephrol. 2013, 45, 1661–1669. [Google Scholar] [CrossRef]
- Narita, T.; Sasaki, H.; Hosoba, M.; Miura, T.; Yoshioka, N.; Morii, T.; Shimotomai, T.; Koshimura, J.; Fujita, H.; Kakei, M.; et al. Parallel Increase in Urinary Excretion Rates of Immunoglobulin G, Ceruloplasmin, Transferrin, and Orosomucoid in Normoalbuminuric Type 2 Diabetic Patients. Diabetes Care 2004, 27, 1176–1181. [Google Scholar] [CrossRef] [Green Version]
- Narita, T.; Hosoba, M.; Kakei, M.; Ito, S. Increased Urinary Excretions of Immunoglobulin G, Ceruloplasmin, and Transferrin Predict Development of Microalbuminuria in Patients with Type 2 Diabetes. Diabetes Care 2006, 29, 142–144. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic Kidney Disease: A Report from an ADA Consensus Conference. Am. J. Kidney Dis. 2014, 64, 510–533. [Google Scholar] [CrossRef] [Green Version]
- Deckert, T.; Feldt-Rasmussen, B.; Djurup, R.; Deckert, M. Glomerular size and charge selectivity in insulin-dependent diabetes mellitus. Kidney Int. 1988, 33, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Jerums, G.; Allen, T.J.; Cooper, M.E. Triphasic Changes in Selectivity with Increasing Proteinuria in Type 1 and Type 2 Diabetes. Diabet. Med. 1989, 6, 772–779. [Google Scholar] [CrossRef]
- Narita, T.; Fujita, H.; Koshimura, J.; Meguro, H.; Kitazato, H.; Shimotomai, T.; Kagaya, E.; Suzuki, K.; Murata, M.; Usami, A.; et al. Glycemic Control Reverses Increases in Urinary Excretions of Immunoglobulin G and Ceruloplasmin in Type 2 Diabetic Patients with Normoalbuminuria. Horm. Metab. Res. 2001, 33, 370–378. [Google Scholar] [CrossRef]
- Deckert, T.; Kofoed-Enevoldsen, A.; Vidal, P.; Andreasen, H.B.; Feldt-Rasmussen, B. Size- and charge selectivity of glomerular filtration in Type 1 (insulin-dependent) diabetic patients with and without albuminuria. Diabetologia 1993, 36, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Scandling, J.D.; Myers, B.D. Glomerular size-selectivity and microalbuminuria in early diabetic glomerular disease. Kidney Int. 1992, 41, 840–846. [Google Scholar] [CrossRef] [Green Version]
- Deen, W.M.; Bridges, C.R.; Brenner, B.M.; Myers, B.D. Heteroporous model of glomerular size selectivity: Application to normal and nephrotic humans. Am. J. Physiol. Physiol. 1985, 249, F374–F389. [Google Scholar] [CrossRef]
- Narita, T.; Kitazato, H.; Koshimura, J.; Suzuki, K.; Murata, M.; Ito, S. Effects of Protein Meals on the Urinary Excretion of Various Plasma Proteins in Healthy Subjects. Nephron 1999, 81, 398–405. [Google Scholar] [CrossRef]
- Hostetter, T.H.; Rennke, H.G.; Brenner, B.M. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am. J. Med. 1982, 72, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Zatz, R.; Meyer, T.W.; Rennke, H.G.; Brenner, B.M. Predominance of hemodynamic rather than metabolic factors in the pathogenesis of diabetic glomerulopathy. Proc. Natl. Acad. Sci. USA 1985, 82, 5963–5967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.; Brenner, B.M. Pathogenesis of diabetic glomerulopathy: Hemodynamic considerations. Diabetes/Metab. Res. Rev. 1988, 4, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Beigi, F.; Craven, T.; Banerji, M.A.; Basile, J.; Calles, J.; Cohen, R.M.; Cuddihy, R.; Cushman, W.C.; Genuth, S.; Grimm, R.H.; et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 2010, 376, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; Grobbee, D.; et al. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [Green Version]
- Zoungas, S.; Chalmers, J.; Ninomiya, T.; Li, Q.; Cooper, M.E.; Colagiuri, S.; Fulcher, G.; De Galan, B.E.; Harrap, S.; Hamet, P.; et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: Evidence of glycaemic thresholds. Diabetologia 2012, 55, 636–643. [Google Scholar] [CrossRef]
- Agrawal, L.; Azad, N.; Emanuele, N.V.; Bahn, G.D.; Kaufman, D.G.; Moritz, T.E.; Duckworth, W.C.; Abraira, C. Observation on Renal Outcomes in the Veterans Affairs Diabetes Trial. Diabetes Care 2011, 34, 2090–2094. [Google Scholar] [CrossRef] [Green Version]
- De Boer, I.H.; Sun, W.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Zinman, B. Intensive Diabetes Therapy and Glomerular Filtration Rate in Type 1 Diabetes. N. Engl. J. Med. 2011, 365, 2366–2376. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Wang, J.; Shen, X.; Lu, W.; Wang, Y.; Li, W.; Gao, Z.; Xu, J.; Li, X.; Liu, R.; et al. Establishment and Validation of a Risk Prediction Model for Early Diabetic Kidney Disease Based on a Systematic Review and Meta-Analysis of 20 Cohorts. Diabetes Care 2020, 43, 925–933. [Google Scholar] [CrossRef]
- Pugliese, G.; Tilton, R.G.; Chang, K.; Speedy, A.; Province, M.; Eades, D.M.; Lacy, P.E.; Kilo, C.; Williamson, J.R. Effects of Islet Isografts on Hemodynamic and Vascular Filtration Changes in Diabetic Rats. Diabetes 1990, 39, 323–332. [Google Scholar] [CrossRef]
- Emara, M.; El-Edel, R.; Fathy, W.M.; Aboelkhair, N.T.; Watany, M.M.; Abou-Elela, D.H. Study the Association of Tumor Necrosis Factor Promoter Polymorphism with Type 2 Diabetic Nephropathy. Mediat. Inflamm. 2020, 2020, 1498278. [Google Scholar] [CrossRef]
- Radcliffe, N.J.; Seah, J.M.; Clarke, M.; MacIsaac, R.J.; Jerums, G.; Ekinci, E.I. Clinical predictive factors in diabetic kidney disease progression. J. Diabetes Investig. 2016, 8, 6–18. [Google Scholar] [CrossRef]
- Russo, G.T.; De Cosmo, S.; Viazzi, F.; Mirijello, A.; Ceriello, A.; Guida, P.; Giorda, C.; Cucinotta, D.; Pontremoli, R.; Fioretto, P. Diabetic kidney disease in the elderly: Prevalence and clinical correlates. BMC Geriatr. 2018, 18, 38. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.K.; Lyles, C.R.; Bent-Shaw, L.A.; Young, B.A. Risk Factor, Age and Sex Differences in Chronic Kidney Disease Prevalence in a Diabetic Cohort: The Pathways Study. Am. J. Nephrol. 2012, 36, 245–251. [Google Scholar] [CrossRef] [Green Version]




| Baseline 24 h UAE < 30 mg/24 h (n = 733) | Baseline 24 h UAE ≥ 30 mg/24 h (n = 302) | |||||
|---|---|---|---|---|---|---|
| Characteristics | Baseline Urine IgG ≤ 2.45 mg/L | Baseline Urine IgG > 2.45 mg/L | p Value | Baseline Urine IgG ≤ 2.45 mg/L | Baseline Urine IgG > 2.45 mg/L | p Value |
| Male (n, %) | 278 (60.4%) | 163 (59.7%) | 0.846 | 20 (46.5%) | 170 (65.6%) | 0.016 |
| Age | 55.91 ± 10.48 | 56.81 ± 10.07 | 0.257 | 56.24 ± 12.04 | 54.34 ± 12.19 | 0.344 |
| BMI (kg/m2) | 26.27 ± 3.65 | 27.02 ± 3.84 | 0.009 | 27.77 ± 4.23 | 27.85 ± 3.84 | 0.908 |
| DM duration (years) | 10.95 ± 6.76 | 10.71 ± 6.89 | 0.656 | 11.92 ± 6.93 | 10.49 ± 7.18 | 0.224 |
| SBP (mmHg) | 130.90 ± 14.83 | 133.78 ± 16.41 | 0.015 | 134.49 ± 15.42 | 137.63 ± 16.77 | 0.252 |
| DBP (mmHg) | 79.29 ± 9.82 | 80.61 ± 9.65 | 0.077 | 82.11 ± 10.40 | 82.43 ± 9.77 | 0.844 |
| HbA1c (%) | 8.41 ± 1.83 | 8.57 ± 1.69 | 0.259 | 8.93 ± 1.91 | 8.83 ± 1.79 | 0.716 |
| eGFR (mL/min/1.73 m2) | 101.02 ± 13.45 | 99.33 ± 13.38 | 0.098 | 105.61 ± 16.22 | 99.23 ± 17.77 | 0.028 |
| ALT (IU/L) | 23.76 ± 14.83 | 23.84 ± 13.43 | 0.945 | 23.27 ± 17.11 | 24.68 ± 16.06 | 0.600 |
| AST (IU/L) | 20.37 ± 8.85 | 20.31 ± 9.59 | 0.930 | 19.37 ± 9.73 | 20.23 ± 9.46 | 0.583 |
| ALT/AST | 1.14 ± 0.40 | 1.17 ± 0.36 | 0.44 | 1.16 ± 0.33 | 1.20 ± 0.43 | 0.547 |
| GGT (U/L) | 22.50 (15.20–37.00) | 26.50 (17.65–37.75) | 0.021 | 26.00 (15.20–38.90) | 25.95 (18.50–40.23) | 0.617 |
| SUA (μmol/L) | 308.30 ± 85.41 | 311.71 ± 85.39 | 0.601 | 328.71 ± 94.00 | 335.42 ± 84.19 | 0.635 |
| TC (mmol/L) | 4.87 ± 1.00 | 5.03 ± 1.29 | 0.064 | 5.35 ± 1.44 | 5.21 ± 1.32 | 0.522 |
| TG (mmol/L) | 1.41 (1.03–2.16) | 1.54 (1.12–2.33) | 0.029 | 1.95 (1.37–3.77) | 1.93 (1.25–3.34) | 0.679 |
| HDL (mmol/L) | 1.25 ± 0.30 | 1.20 ± 0.26 | 0.048 | 1.18 ± 0.26 | 1.18 ± 0.27 | 0.978 |
| LDL (mmol/L) | 3.06 ± 0.81 | 3.09 ± 1.05 | 0.062 | 3.34 ± 1.18 | 3.25 ± 1.00 | 0.597 |
| Smoking (n, %) | 9(2.0%) | 3(1.1%) | 0.560 | 0(0.0%) | 6(2.3%) | 0.599 |
| Retinopathy (n, %) | 185 (40.2%) | 102 (37.4%) | 0.444 | 15 (34.9%) | 115 (44.4%) | 0.243 |
| ACEI/ARB use (n, %) | 12(2.6%) | 10(3.7%) | 0.419 | 4(9.3%) | 12(4.6%) | 0.369 |
| Statin use (n, %) | 4(0.9%) | 1(0.4%) | 0.656 | 0(0.0%) | 3(1.2%) | 1.000 |
| Urine IgG (mg/L) | 0.68 (0.21–1.36) | 5.10 (3.44–7.98) | <0.001 | 1.14 (0.58–1.78) | 11.98 (6.46–27.64) | <0.001 |
| Urine RBP (mg/L) | 0.15 (0.06–0.32) | 0.37 (0.18–0.68) | <0.001 | 0.18 (0.11–0.32) | 0.77 (0.29–1.61) | <0.001 |
| Urine β2-MG (mg/L) | 0.10 (0.06–0.18) | 0.16 (0.09–0.38) | <0.001 | 0.11 (0.07–0.26) | 0.16 (0.08–0.46) | 0.07 |
| 24 h UAE (mg/24 h) | 11.05 (5.29–15.00) | 14.30 (10.15–20.35) | <0.001 | 55.38 (41.14–74.24) | 87.70 (52.65–149.20) | <0.001 |
| Follow-up time (years) | 4.16 ± 0.88 | 4.23 ± 0.85 | 0.303 | 4.45 ± 0.92 | 4.28 ± 0.94 | 0.288 |
| Baseline 24 h UAE < 30 mg/24 h (n = 733) | Baseline 24 h UAE ≥ 30 mg/24 h (n = 302) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Baseline Urine IgG ≤ 2.45 mg/L | Baseline Urine IgG > 2.45 mg/L | χ2 | p-Value | Outcomes | Baseline Urine IgG ≤ 2.45 mg/L | Baseline Urine IgG > 2.45 mg/L | χ2 | p-Value |
| No Onset (n = 595) | 402 (67.6%) | 193 (32.4%) | 39.565 | <0.001 | Nonprogress (n = 207) | 40 (19.3%) | 167 (80.7%) | 14.104 | 0.003 |
| Onset1 (n = 14) | 10 (71.4%) | 4 (28.6%) | Progress1 (n = 15) | 1 (6.7%) | 14 (93.3%) | ||||
| Onset2 (n = 109) | 45 (41.3%) | 64 (58.7%) | Progress2 (n = 58) | 2 (3.4%) | 56 (96.6%) | ||||
| Onset3 (n = 15) | 3 (20.0%) | 12 (80.0%) | Progress3 (n = 22) | 0 (0.0%) | 22 (100.0%) | ||||
| Baseline 24 h UAE < 30 mg/24 h (n = 733) | Baseline 24 h UAE ≥ 30 mg/24 h (n = 302) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||||
| Outcomes | Baseline Urine IgG | OR (95% CI) | p-Value | OR (95% CI) | p-Value a | Outcomes | Baseline Urine IgG | OR (95% CI) | p-Value | OR (95% CI) | p-Value a |
| Onset-1 b (n = 14) | Urine IgG > 2.45 mg/L vs.Urine IgG ≤ 2.45 mg/L | 0.833 (0.258–2.688) | 0.760 | 0.609 (0.121–3.067) | 0.548 | Progress1 c (n = 15) | Urine IgG > 2.45 mg/L vs.Urine IgG ≤ 2.45 mg/L | 3.115 (0.396–24.390) | 0.281 | 3.115 (0.180–52.631) | 0.434 |
| Onset-2 b (n = 109) | Urine IgG > 2.45 mg/L vs.Urine IgG ≤ 2.45 mg/L | 2.959 (1.949–4.505) | <0.001 | 2.617 (1.623–4.219) | <0.001 | Progress2 c (n = 58) | Urine IgG > 2.45 mg/L vs.Urine IgG ≤ 2.45 mg/L | 6.711 (1.570–28.571) | 0.010 | 7.353 (1.475–37.037) | 0.015 |
| Onset-3 b (n = 15) | Urine IgG > 2.45 mg/L vs.Urine IgG ≤ 2.45 mg/L | 8.333 (2.326–30.303) | <0.001 | 14.706 (2.188–100.000) | 0.006 | Progress3 c (n = 22) | Urine IgG > 2.45 mg/L vs.Urine IgG ≤ 2.45 mg/L | — | — | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C.; Chen, J.; Sun, X.; Guan, S.; Zhu, H.; Qin, Y.; Wang, J.; Li, Y.; Yang, J.; Chang, B. Urine Immunoglobin G Greater Than 2.45 mg/L Has a Correlation with the Onset and Progression of Diabetic Kidney Disease: A Retrospective Cohort Study. J. Pers. Med. 2023, 13, 452. https://doi.org/10.3390/jpm13030452
Meng C, Chen J, Sun X, Guan S, Zhu H, Qin Y, Wang J, Li Y, Yang J, Chang B. Urine Immunoglobin G Greater Than 2.45 mg/L Has a Correlation with the Onset and Progression of Diabetic Kidney Disease: A Retrospective Cohort Study. Journal of Personalized Medicine. 2023; 13(3):452. https://doi.org/10.3390/jpm13030452
Chicago/Turabian StyleMeng, Cheng, Jiujing Chen, Xiaoyue Sun, Shilin Guan, Hong Zhu, Yongzhang Qin, Jingyu Wang, Yongmei Li, Juhong Yang, and Baocheng Chang. 2023. "Urine Immunoglobin G Greater Than 2.45 mg/L Has a Correlation with the Onset and Progression of Diabetic Kidney Disease: A Retrospective Cohort Study" Journal of Personalized Medicine 13, no. 3: 452. https://doi.org/10.3390/jpm13030452
APA StyleMeng, C., Chen, J., Sun, X., Guan, S., Zhu, H., Qin, Y., Wang, J., Li, Y., Yang, J., & Chang, B. (2023). Urine Immunoglobin G Greater Than 2.45 mg/L Has a Correlation with the Onset and Progression of Diabetic Kidney Disease: A Retrospective Cohort Study. Journal of Personalized Medicine, 13(3), 452. https://doi.org/10.3390/jpm13030452