The Role of Folic Acid in SARS-CoV-2 Infection: An Intriguing Linkage under Investigation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
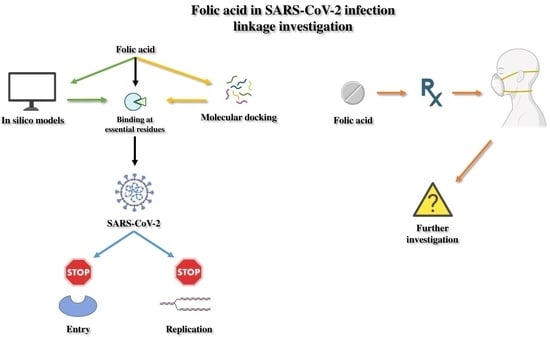
3.1. In Silico Studies and Molecular Docking Concerning Folic Acid and SARS-CoV-2 Potential Linkage
3.2. Patients’ Studies Investigating the Interplay between SARS-CoV-2 and Folic Acid Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giovanetti, M.; Benedetti, F.; Campisi, G.; Ciccozzi, A.; Fabris, S.; Ceccarelli, G.; Tambone, V.; Caruso, A.; Angeletti, S.; Zella, D.; et al. Evolution patterns of SARS-CoV-2: Snapshot on its genome variants. Biochem. Biophys. Res. Commun. 2021, 538, 88–91. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Dashboard. 2023. Available online: https://covid19.who.int/?adgroupsurvey={adgroupsurvey}&gclid=Cj0KCQiA6rCgBhDVARIsAK1kGPJShqhSazK69kE9YV6yPh6XwE3_iOpfk5OmWdkgKRVXM7xnA96GSD4aAjJyEALw_wcB (accessed on 11 March 2023).
- Bakhiet, M.; Taurin, S. SARS-CoV-2: Targeted managements and vaccine development. Cytokine Growth Factor Rev. 2021, 58, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Jahanafrooz, Z.; Abbasi, A.; Goli, M.B.; Sadeghi, M.; Mottaqi, M.S.; Zamani, M. Mutations in SARS-CoV-2; Consequences in structure, function, and pathogenicity of the virus. Microb. Pathog. 2021, 154, 104831. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell. Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e899. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zhang, W. SARS-CoV-2 variants, immune escape, and countermeasures. Front. Med. 2022, 16, 196–207. [Google Scholar] [CrossRef]
- Meo, S.A.; Meo, A.S.; Al-Jassir, F.F.; Klonoff, D.C. Omicron SARS-CoV-2 new variant: Global prevalence and biological and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 8012–8018. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.; Pawar, S.V.; Kumar, A. A Review on Repurposed Drugs and Vaccine Trials for Combating SARS CoV-2. Curr. Drug Res. Rev. 2021, 13, 203–221. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Patti, G.; Pellegrino, C.; Ricciardi, A.; Novara, R.; Cotugno, S.; Papagni, R.; Guido, G.; Totaro, V.; De Iaco, G.; Romanelli, F.; et al. Potential Role of Vitamins A, B, C, D and E in TB Treatment and Prevention: A Narrative Review. Antibiotics 2021, 10, 1354. [Google Scholar] [CrossRef]
- Papagni, R.; Pellegrino, C.; Di Gennaro, F.; Patti, G.; Ricciardi, A.; Novara, R.; Cotugno, S.; Musso, M.; Guido, G.; Ronga, L.; et al. Impact of Vitamin D in Prophylaxis and Treatment in Tuberculosis Patients. Int. J. Mol. Sci. 2022, 23, 3860. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Sobczynska-Malefora, A. The adverse effects of an excessive folic acid intake. Eur. J. Clin. Nutr. 2017, 71, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Golob, J.L.; Lugogo, N.; Lauring, A.S.; Lok, A.S. SARS-CoV-2 vaccines: A triumph of science and collaboration. JCI Insight 2021, 6, e149187. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Caminada, S.; Isonne, C.; Santoro, M.M.; Baccolini, V. What Are the Effects of Vitamin A Oral Supplementation in the Prevention and Management of Viral Infections? A Systematic Review of Randomized Clinical Trials. Nutrients 2022, 1, 4081. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Hwang, Y.C.; Lu, R.M.; Su, S.C.; Chiang, P.Y.; Ko, S.H.; Ke, F.Y.; Liang, K.H.; Hsieh, T.Y.; Wu, H.C. Monoclonal antibodies for COVID-19 therapy and SARS-CoV-2 detection. J. Biomed. Sci. 2022, 29, 1. [Google Scholar] [CrossRef]
- Gazzali, A.M.; Lobry, M.; Colombeau, L.; Acherar, S.; Azais, H.; Mordon, S.; Arnoux, P.; Baros, F.; Vanderesse, R.; Frochot, C. Stability of folic acid under several parameters. Eur. J. Pharm. Sci. 2016, 93, 419–430. [Google Scholar] [CrossRef]
- Sijilmassi, O. Folic acid deficiency and vision: A review. Graefes. Arch. Clin. Exp. Ophthalmol. 2019, 257, 1573–1580. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Naderi, N.; House, J.D. Recent Developments in Folate Nutrition. Adv. Food Nutr. Res. 2018, 83, 195–213. [Google Scholar] [CrossRef]
- Mount Sinai Medical System Website. Available online: https://www.mountsinai.org/health-library/tests/folic-acid-test (accessed on 11 March 2023).
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic acid versus 5-methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Balashova, O.A.; Visina, O.; Borodinsky, L.N. Folate action in nervous system development and disease. Dev. Neurobiol. 2018, 78, 391–402. [Google Scholar] [CrossRef]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef] [Green Version]
- Socha, D.S.; DeSouza, S.I.; Flagg, A.; Sekeres, M.; Rogers, H.J. Severe megaloblastic anemia: Vitamin deficiency and other causes. Cleve Clin. J. Med. 2020, 87, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Van Gool, J.D.; Hirche, H.; Lax, H.; De Schaepdrijver, L. Folic acid and primary prevention of neural tube defects: A review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Sarma, P.; Bhattacharyya, A.; Prajapat, M.; Kumar, S.; Prakash, A.; Medhi, B. Folic acid as placebo in controlled clinical trials of hydroxychloroquine prophylaxis in COVID-19: Is it scientifically justifiable? Med. Hypotheses 2021, 149, 110539. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, J.L.; Qin, R.S.; Hou, J.P.; Zang, G.C.; Zhang, G.Y.; Chen, T.T. Folic acid: A potential inhibitor against SARS-CoV-2 nucleocapsid protein. Pharm. Biol. 2022, 60, 862–878. [Google Scholar] [CrossRef]
- Ugurel, O.M.; Mutlu, O.; Sariyer, E.; Kocer, S.; Ugurel, E.; Inci, T.G.; Ata, O.; Turgut-Balik, D. Evaluation of the potency of FDA-approved drugs on wild type and mutant SARS-CoV-2 helicase (Nsp13). Int. J. Biol. Macromol. 2020, 163, 1687–1696. [Google Scholar] [CrossRef]
- Serseg, T.; Benarous, K.; Yousfi, M. Hispidin and Lepidine E: Two Natural Compounds and Folic Acid as Potential Inhibitors of 2019-novel Coronavirus Main Protease (2019-nCoVM (pro)), Molecular Docking and SAR Study. Curr. Comput. Aided Drug Des. 2021, 17, 469–479. [Google Scholar] [CrossRef]
- Kumar, V.; Kancharla, S.; Jena, M.K. In silico virtual screening-based study of nutraceuticals predicts the therapeutic potentials of folic acid and its derivatives against COVID-19. Virusdisease 2021, 32, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, V. Repurposing the natural compounds as potential therapeutic agents for COVID-19 based on the molecular docking study of the main protease and the receptor-binding domain of spike protein. J. Mol. Model 2022, 28, 153. [Google Scholar] [CrossRef]
- Acosta-Elias, J.; Espinosa-Tanguma, R. The Folate Concentration and/or Folic Acid Metabolites in Plasma as Factor for COVID-19 Infection. Front. Pharmacol. 2020, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Topless, R.; Green, R.; Morgan, S.L.; Robinson, P.; Merriman, T.; Gaffo, A.L. Folic acid and methotrexate use and their association with COVID-19 diagnosis and mortality: A case-control analysis from the UK Biobank. BMJ Open 2022, 12, e062945. [Google Scholar] [CrossRef] [PubMed]
- Bliek-Bueno, K.; Mucherino, S.; Poblador-Plou, B.; Gonzalez-Rubio, F.; Aza-Pascual-Salcedo, M.; Orlando, V.; Clerencia-Sierra, M.; Ioakeim-Skoufa, I.; Coscioni, E.; Carmona-Pirez, J.; et al. Baseline Drug Treatments as Indicators of Increased Risk of COVID-19 Mortality in Spain and Italy. Int. J. Environ. Res. Public Health 2021, 18, 11786. [Google Scholar] [CrossRef] [PubMed]
- Colquitt, R.B.; Colquhoun, D.A.; Thiele, R.H. In silico modelling of physiologic systems. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 499–510. [Google Scholar] [CrossRef]
- Yuriev, E.; Agostino, M.; Ramsland, P.A. Challenges and advances in computational docking: 2009 in review. J. Mol. Recognit. 2011, 24, 149–164. [Google Scholar] [CrossRef]
- Adhikari, P.M.; Chowta, M.N.; Ramapuram, J.T.; Rao, S.B.; Udupa, K.; Acharya, S.D. Effect of Vitamin B12 and folic acid supplementation on neuropsychiatric symptoms and immune response in HIV-positive patients. J. Neurosci. Rural Pract. 2016, 7, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lian, J.; Yi, L.; Yao, T.; Feng, S.; Wang, B.; Li, J.; Feng, Y.; Wang, S. Folic acid supplementation in pregnant women with hepatitis B surface antigen improves infant hepatitis B surface antibody mediated by infant interleukin-4. Br. J. Nutr. 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pijeira, M.S.O.; de Menezes, A.S.; Fechine, P.B.A.; Shah, S.Q.; Ilem-Ozdemir, D.; Lopez, E.O.; Maricato, J.T.; Rosa, D.S.; Ricci-Junior, E.; Junior, S.A.; et al. Folic acid-functionalized graphene quantum dots: Synthesis, characterization, radiolabeling with radium-223 and antiviral effect against Zika virus infection. Eur. J. Pharm. Biopharm. 2022, 180, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yenigul, N.N.; Yazici Yilmaz, F.; Ayhan, I. Can Serum Vitamin B12 and Folate Levels Predict HPV Penetration in Patients with ASCUS? Nutr. Cancer 2021, 73, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Abike, F.; Engin, A.B.; Dunder, I.; Tapisiz, O.L.; Aslan, C.; Kutluay, L. Human papilloma virus persistence and neopterin, folate and homocysteine levels in cervical dysplasias. Arch. Gynecol. Obstet. 2011, 284, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Empig, C.J.; Welte, F.J.; Speck, R.F.; Schmaljohn, A.; Kreisberg, J.F.; Goldsmith, M.A. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 2001, 106, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Empig, C.J.; Goldsmith, M.A. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 2002, 76, 5266–5270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilaseca, M.A.; Sierra, C.; Colome, C.; Artuch, R.; Valls, C.; Munoz-Almagro, C.; Vilches, M.A.; Fortuny, C. Hyperhomocysteinaemia and folate deficiency in human immunodeficiency virus-infected children. Eur. J. Clin. Investig. 2001, 31, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Powers, H.J. Interaction among folate, riboflavin, genotype, and cancer, with reference to colorectal and cervical cancer. J. Nutr. 2005, 135, 2960S–2966S. [Google Scholar] [CrossRef] [Green Version]
- Bertacine Dias, M.V.; Santos, J.C.; Libreros-Zuniga, G.A.; Ribeiro, J.A.; Chavez-Pacheco, S.M. Folate biosynthesis pathway: Mechanisms and insights into drug design for infectious diseases. Future Med. Chem. 2018, 10, 935–959. [Google Scholar] [CrossRef]
- Ye, Z.Q.; Zou, C.L.; Chen, H.B.; Lv, Q.Y.; Wu, R.Q.; Gu, D.N. Folate-conjugated herpes simplex virus for retargeting to tumor cells. J. Gene Med. 2020, 22, e3177. [Google Scholar] [CrossRef]

| Author/[Ref] | Year of Study | Study Design | Main Outcomes |
|---|---|---|---|
| Chen et al./[30] | 2022 | Target drugs, hub genes, molecular docking | FA was potentially an antagonist of SARS-CoV-2 N, but its effect on viruses is unclear |
| Ugurel et al./[31] | 2020 | Genome sequences, protein–drug interactions, in silico methods | FA was among the most promising drugs, having the potency to inhibit both the wild type and mutant SARS-CoV-2 helicase |
| Serseg et al./[32] | 2021 | Molecular docking | Hispidin, lepidine E, and FA inhibited the main protease of the virus |
| Kumar et al./[33] | 2021 | Molecular docking | FA alone, or in combination with its derivates, such as tetrahydrofolic acid and 5-methyl tetrahydrofolic acid, were potential molecules against COVID-19 infection |
| Eskandari/[34] | 2022 | Molecular docking, in silico methods | FA, among other vitamins, inhibited SARS-CoV-2 entry into the host and viral replication, binding at important residues |
| Author/[Ref] | Year | Study Design | Study Population | Main Findings |
|---|---|---|---|---|
| Acosta-Elias et al./[35] | 2020 | Comparative study | Pregnant vs non pregnant women suffering from influenza vs COVID-19 | Protective role of FA against SARS-CoV-2 infection |
| Topless et al./[36] | 2022 | Case control analysis | Data from 380.380 UKBB participants with general practice prescription data for 2019–2021 | Relation between COVID-19 diagnosis, COVID-19-related death, and FA oral prescription. Methotrexate diminished the relation |
| Bliek-Buen et al./[37] | 2021 | Retrospective, observational study | An amount of 8570 subjects, from two regions in Spain and Italy | Oral FA supplementationwas significantly associated with 30-day mortality in subjects with COVID-19 in both regions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakousis, N.D.; Gourgoulianis, K.I.; Kotsiou, O.S. The Role of Folic Acid in SARS-CoV-2 Infection: An Intriguing Linkage under Investigation. J. Pers. Med. 2023, 13, 561. https://doi.org/10.3390/jpm13030561
Karakousis ND, Gourgoulianis KI, Kotsiou OS. The Role of Folic Acid in SARS-CoV-2 Infection: An Intriguing Linkage under Investigation. Journal of Personalized Medicine. 2023; 13(3):561. https://doi.org/10.3390/jpm13030561
Chicago/Turabian StyleKarakousis, Nikolaos D., Konstantinos I. Gourgoulianis, and Ourania S. Kotsiou. 2023. "The Role of Folic Acid in SARS-CoV-2 Infection: An Intriguing Linkage under Investigation" Journal of Personalized Medicine 13, no. 3: 561. https://doi.org/10.3390/jpm13030561
APA StyleKarakousis, N. D., Gourgoulianis, K. I., & Kotsiou, O. S. (2023). The Role of Folic Acid in SARS-CoV-2 Infection: An Intriguing Linkage under Investigation. Journal of Personalized Medicine, 13(3), 561. https://doi.org/10.3390/jpm13030561