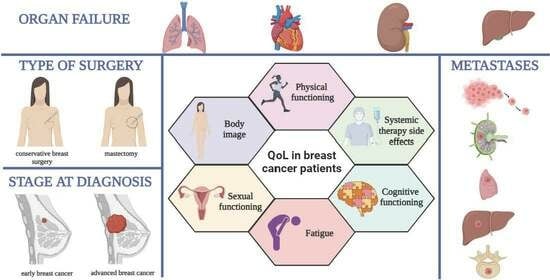
Assessing the Impact of Organ Failure and Metastases on Quality of Life in Breast Cancer Patients: A Prospective Study Based on Utilizing EORTC QLQ-C30 and EORTC QLQ-BR45 Questionnaires in Romania
Abstract
:1. Introduction
2. Materials and Methods
- Female patients who were treated solely in the Colțea Clinical Hospital;
- Patients aged over 18 years diagnosed with stage 0–IV breast cancer by histopathological examination.
- Male patients;
- Patients who did not give their consent to participate in this study;
- Patients in visceral crisis;
- Patients diagnosed with premalignant breast cancer lesions;
- Patients who became deceased during data collection;
- Patients who presented other types of malignancy.
2.1. Ethical Considerations
2.2. Statistical Analysis
2.3. Study Limitations
3. Results
4. Discussion
4.1. Survival Analysis
4.2. Analysis of QoL Results Using EORTC QLQ-C30 and EORTC QLQ-BR45 Questionnaires
4.3. Age and Menopausal Status
4.4. Cancer Staging
4.5. Type of Surgery
4.6. Quality of Life of Patients with Breast Cancer and Organ Failure
4.6.1. Heart Failure
4.6.2. Renal Failure
4.6.3. Neurologic Dysfunction
4.6.4. Liver Failure
4.6.5. Respiratory Failure
4.7. Patients with Osseous and Non-Osseous Metastases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A








References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Shulman, L.N.; Willett, W.; Sievers, A.; Knaul, F.M. Breast Cancer in Developing Countries: Opportunities for Improved Survival. J. Oncol. 2010, 2010, 595167. [Google Scholar] [CrossRef]
- Da Costa Vieira, R.A.; Biller, G.; Uemura, G.; Ruiz, C.A.; Curado, M.P. Breast Cancer Screening in Developing Countries. Clinics 2017, 72, 244–253. [Google Scholar] [CrossRef]
- Lv, L.; Zhao, B.; Kang, J.; Li, S.; Wu, H. Trend of Disease Burden and Risk Factors of Breast Cancer in Developing Countries and Territories, from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Front. Public Health 2022, 10, 1078191. [Google Scholar] [CrossRef]
- Boring, C.C.; Squires, T.S.; Tong, T.; Montgomery, S. Cancer Statistics, 1994. CA A Cancer J. Clin. 1994, 44, 7–26. [Google Scholar] [CrossRef] [PubMed]
- WHOQOL—Measuring Quality of Life. The World Health Organization. Available online: https://www.who.int/tools/whoqol (accessed on 20 January 2024).
- Clarijs, M.E.; Thurell, J.; Kühn, F.; Uyl-de Groot, C.A.; Hedayati, E.; Karsten, M.M.; Jager, A.; Koppert, L.B. Measuring Quality of Life Using Patient-Reported Outcomes in Real-World Metastatic Breast Cancer Patients: The Need for a Standardized Approach. Cancers 2021, 13, 2308. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.F.; Cherin, E.A. Quality of Life during and after Treatment. Compr. Ther. 1988, 14, 69–75. [Google Scholar]
- Psychological Tests and Scales. In CiNii Research. Available online: https://cir.nii.ac.jp/crid/1571980074418283776 (accessed on 21 January 2024).
- Shumaker, S.A.; Wyman, J.F.; Uebersax, J.S.; McClish, D.; Fantl, J.A. Health-Related Quality of Life Measures for Women with Urinary Incontinence: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Qual. Life Res. 1994, 3, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Romney, D.M.; Brown, R.I.; Fry, P.S. Improving the Quality of Life: Recommendations for People with and without Disabilities; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994; ISBN 978-0-7923-3234-3. [Google Scholar]
- Fayers, P.; Bottomley, A.; EORTC Quality of Life Group; Quality of Life Unit Quality of Life Research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur. J. Cancer 2002, 38 (Suppl. S4), S125–S133. [Google Scholar] [CrossRef] [PubMed]
- Bjelic-Radisic, V.; Cardoso, F.; Cameron, D.; Brain, E.; Kuljanic, K.; da Costa, R.A.; Conroy, T.; Inwald, E.C.; Serpentini, S.; Pinto, M.; et al. An International Update of the EORTC Questionnaire for Assessing Quality of Life in Breast Cancer Patients: EORTC QLQ-BR45. Ann. Oncol. 2020, 31, 283–288. [Google Scholar] [CrossRef]
- Brady, M.J.; Cella, D.F.; Mo, F.; Bonomi, A.E.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and Validity of the Functional Assessment of Cancer Therapy-Breast Quality-of-Life Instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef]
- Ripamonti, C.I.; Bandieri, E.; Pessi, M.A.; Maruelli, A.; Buonaccorso, L.; Miccinesi, G. The Edmonton Symptom Assessment System (ESAS) as a Screening Tool for Depression and Anxiety in Non-Advanced Patients with Solid or Haematological Malignancies on Cure or Follow-Up. Support. Care Cancer 2014, 22, 783–793. [Google Scholar] [CrossRef]
- De Oliveira, L.L.E.; da Silva, M.M. Quality of Life of Women with Locally Advanced or Metastatic Breast Cancer. Rev. Gauch. Enferm. 2020, 41, e20190292. [Google Scholar] [CrossRef]
- Groza, A.; Iconaru, S.L.; Jiga, G.; Chapon, P.; Gaiaschi, S.; Verga, N.; Beuran, M.; Prodan, A.M.; Matei, M.; Marinescu, S.A.; et al. The Effect of the Ionizing Radiation on Hydroxyapatite–Polydimethylsiloxane Layers. Polym. Eng. Sci. 2019, 59, 2406–2412. [Google Scholar] [CrossRef]
- Coniac, S.; Costache Outas, M.C.; Pirvu, E.-E.; Patru, R.-I.; Gainariu, E.; Aldea, C.; Iorga, P.G.; Ambroci, M.; Liscu, H.-D.; Miron, A.-I.; et al. Challenges and Limitations of Endocrine Toxicity Evaluation in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy—Retrospective Study from a Tertiary-Level Hospital in Romania. Diagnostics 2023, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.-I.; Anghel, A.-V.; Barnonschi, A.-A.; Mitre, R.; Liscu, H.-D.; Găinariu, E.; Pătru, R.; Coniac, S. Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania. Diagnostics 2023, 13, 1938. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Bhati, R.S.; von Rottenthaler, E.E.; Reagan, A.M.; Karver, S.B.; Reich, R.R.; Quinn, G.P. Therapy Choices and Quality of Life in Young Breast Cancer Survivors: A Short-Term Follow-Up. Am. J. Surg. 2013, 206, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Quality of Life, Fertility Concerns, and Behavioral Health Outcomes in Younger Breast Cancer Survivors: A Systematic Review—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22271773/ (accessed on 20 January 2024).
- Quality of Life, Clinical Trials, and Clinical Practice. Available online: https://connection.asco.org/blogs/quality-life-clinical-trials-and-clinical-practice (accessed on 20 January 2024).
- Weldring, T.; Smith, S.M.S. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv. Insights 2013, 6, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.E.; Huang, P. Cautionary Note Regarding the Use of CIs Obtained From Kaplan-Meier Survival Curves. J. Clin. Oncol. 2009, 27, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Global Surveillance of Trends in Cancer Survival: Analysis of Individual Records for 37,513,025 Patients Diagnosed with One of 18 Cancers during 2000–2014 from 322 Population-Based Registries in 71 Countries (CONCORD-3)—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5879496/ (accessed on 24 January 2024).
- Mangone, L.; Marinelli, F.; Bisceglia, I.; Braghiroli, M.B.; Damato, A.; Pinto, C. Five-Year Relative Survival by Stage of Breast and Colon Cancers in Northern Italy. Front. Oncol. 2022, 12, 982461. [Google Scholar] [CrossRef]
- Survival of Breast Cancer by Stage, Grade and Molecular Groups in Mallorca, Spain—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9571737/ (accessed on 24 January 2024).
- Imran, M.; Al-Wassia, R.; Alkhayyat, S.S.; Baig, M.; Al-Saati, B.A. Assessment of Quality of Life (QoL) in Breast Cancer Patients by Using EORTC QLQ-C30 and BR-23 Questionnaires: A Tertiary Care Center Survey in the Western Region of Saudi Arabia. PLoS ONE 2019, 14, e0219093. [Google Scholar] [CrossRef]
- Jassim, G.A.; Whitford, D.L. Quality of Life of Bahraini Women with Breast Cancer: A Cross Sectional Study. BMC Cancer 2013, 13, 212. [Google Scholar] [CrossRef]
- Roman, A.-M.; Petca, R.-C.; Dumitrașcu, M.C.; Petca, A.; Ionescu (Miron), A.-I.; Șandru, F. Frontal Fibrosing Alopecia and Reproductive Health: Assessing the Role of Sex Hormones in Disease Development. J. Pers. Med. 2024, 14, 72. [Google Scholar] [CrossRef]
- Castillo, H.; Mension, E. Sexual Function in Breast Cancer Patients: A Review of the Literature. Clin. Exp. Obstet. Gynecol. 2022, 49, 134. [Google Scholar] [CrossRef]
- Marschner, N.; Trarbach, T.; Rauh, J.; Meyer, D.; Müller-Hagen, S.; Harde, J.; Dille, S.; Kruggel, L.; Jänicke, M.; The TMK-Group (Tumour Registry Breast Cancer). Quality of Life in Pre- and Postmenopausal Patients with Early Breast Cancer: A Comprehensive Analysis from the Prospective MaLife Project. Breast Cancer Res. Treat. 2019, 175, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Sandru, F.; Petca, R.-C.; Costescu, M.; Dumitrașcu, M.C.; Popa, A.; Petca, A.; Miulescu, R.-G. Cutaneous Mastocytosis in Childhood—Update from the Literature. J. Clin. Med. 2021, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- De Mello Ramirez Medina, J.; de Araujo Trugilho, I.; Mendes, G.N.B.; Silva, J.G.; da Silva Paiva, M.A.; de Aguiar, S.S.; Thuler, L.C.S.; Bergmann, A. Advanced Clinical Stage at Diagnosis of Breast Cancer Is Associated with Poorer Health-Related Quality of Life: A Cross-Sectional Study. Eur. J. Breast Health 2019, 15, 26–31. [Google Scholar] [CrossRef] [PubMed]
- King, M.T.; Winters, Z.E.; Olivotto, I.A.; Spillane, A.J.; Chua, B.H.; Saunders, C.; Westenberg, A.H.; Mann, G.B.; Burnett, P.; Butow, P.; et al. Patient-Reported Outcomes in Ductal Carcinoma in Situ: A Systematic Review. Eur. J. Cancer 2017, 71, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.; Bovbjerg, D.; Schwartz, M.; Hudis, C.; Gilewski, T.; Norton, L. Conditioned Emotional Distress in Women Receiving Chemotherapy for Breast Cancer. J. Consult. Clin. Psychol. 1995, 63, 108–114. [Google Scholar] [CrossRef]
- Broeckel, J.A.; Jacobsen, P.B.; Horton, J.; Balducci, L.; Lyman, G.H. Characteristics and Correlates of Fatigue after Adjuvant Chemotherapy for Breast Cancer. J. Clin. Oncol. 1998, 16, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Weitzner, M.A.; Meyers, C.A.; Stuebing, K.K.; Saleeba, A.K. Relationship between Quality of Life and Mood in Long-Term Survivors of Breast Cancer Treated with Mastectomy. Support. Care Cancer 1997, 5, 241–248. [Google Scholar] [CrossRef]
- Quality of Life, Depression, and Stress in Breast Cancer Women Outpatients Receiving Active Therapy in Taiwan—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16594937/ (accessed on 21 January 2024).
- Akça, M.; Ata, A.; Nayır, E.; Erdoğdu, S.; Arıcan, A. Impact of Surgery Type on Quality of Life in Breast Cancer Patients. J. Breast Health 2014, 10, 222–228. [Google Scholar] [CrossRef] [PubMed]
- De Ligt, K.M.; Heins, M.; Verloop, J.; Ezendam, N.P.M.; Smorenburg, C.H.; Korevaar, J.C.; Siesling, S. The Impact of Health Symptoms on Health-Related Quality of Life in Early-Stage Breast Cancer Survivors. Breast Cancer Res. Treat. 2019, 178, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Quality of Life, Problems, and Needs of Disease-Free Breast Cancer Survivors 5 Years after Diagnosis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29740782/ (accessed on 21 January 2024).
- Depression in Breast Cancer Patients Who Have Undergone Mastectomy: A National Cohort Study—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28394909/ (accessed on 21 January 2024).
- Sexual Functioning in Women after Mastectomy versus Breast Conserving Therapy for Early-Stage Breast Cancer: A Prospective Controlled Study—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25082211/ (accessed on 21 January 2024).
- Molavi, A.; Hekmat, K.; Afshari, P.; Hoseini, M. Evaluation of Couples’ Sexual Function and Satisfaction after Mastectomy. Iran. J. Obstet. Gynecol. Infertil. 2015, 17, 17–23. [Google Scholar]
- Neto, M.S.; de Aguiar Menezes, M.V.; Moreira, J.R.; Garcia, E.B.; Abla, L.E.F.; Ferreira, L.M. Sexuality after Breast Reconstruction Post Mastectomy. Aesthetic Plast. Surg. 2013, 37, 643–647. [Google Scholar] [CrossRef]
- Nesvold, I.-L.; Dahl, A.A.; Løkkevik, E.; Marit Mengshoel, A.; Fosså, S.D. Arm and Shoulder Morbidity in Breast Cancer Patients after Breast-Conserving Therapy versus Mastectomy. Acta Oncol. 2008, 47, 835–842. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Northfelt, D.W.; Gentile, F.; Zamorano, J.L.; Khandheria, B.K. Trastuzumab-Induced Cardiotoxicity: Heart Failure at the Crossroads. Mayo Clin. Proc. 2008, 83, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ghuloom, A.M.; Sanad, H.M. Perceived Quality of Life in Patients with Heart Failure: A Cross-Sectional Study among Adults in Kingdom of Bahrain. Arab. Gulf J. Sci. Res. 2022, 41, 67–76. [Google Scholar] [CrossRef]
- Marital Status and Outcomes in Patients With Cardiovascular Disease—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29263033/ (accessed on 21 January 2024).
- Tromp, J.; Shen, L.; Jhund, P.S.; Anand, I.S.; Carson, P.E.; Desai, A.S.; Granger, C.B.; Komajda, M.; McKelvie, R.S.; Pfeffer, M.A.; et al. Age-Related Characteristics and Outcomes of Patients With Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2019, 74, 601–612. [Google Scholar] [CrossRef]
- Cai, J.-H.; Zheng, J.-H.; Lin, X.-Q.; Lin, W.-X.; Zou, J.; Chen, Y.-K.; Li, Z.-Y.; Chen, Y.-X. Individualized Treatment of Breast Cancer with Chronic Renal Failure: A Case Report and Review of Literature. World J. Clin. Cases 2021, 9, 10345–10354. [Google Scholar] [CrossRef]
- Liu, W.; Peng, J.; Tang, M. Individualized Treatment Analysis of Breast Cancer With Chronic Renal Failure. Onco Targets Ther. 2019, 12, 7767–7772. [Google Scholar] [CrossRef] [PubMed]
- Modi, G.K.; Jha, V. The Incidence of End-Stage Renal Disease in India: A Population-Based Study. Kidney Int. 2006, 70, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Sefko, J.A.; Jeffe, D.B.; Schootman, M. The Association between Chronic Disease Burden and Quality of Life among Breast Cancer Survivors in Missouri. Breast Cancer Res. Treat. 2011, 129, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.G.; Yurter, A.; Gokaslan, Z.L.; Sciubba, D.M. Diagnosis and Surgical Management of Breast Cancer Metastatic to the Spine. World J. Clin. Oncol. 2014, 5, 263–271. [Google Scholar] [CrossRef] [PubMed]
- The Surgical Management of Metastatic Epidural Compression of the Spinal Cord—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20675746/ (accessed on 21 January 2024).
- The Tokuhashi Score: Effectiveness and Pitfalls—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26626082/ (accessed on 21 January 2024).
- Lacourt, T.E.; Heijnen, C.J. Mechanisms of Neurotoxic Symptoms as a Result of Breast Cancer and Its Treatment: Considerations on the Contribution of Stress, Inflammation, and Cellular Bioenergetics. Curr. Breast Cancer Rep. 2017, 9, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Ristea, N.-C.; Miron, A.-I.; Savencu, O.; Georgescu, M.-I.; Verga, N.; Khan, F.S.; Ionescu, R.T. CyTran: A Cycle-Consistent Transformer with Multi-Level Consistency for Non-Contrast to Contrast CT Translation. Neurocomputing 2023, 538, 126211. [Google Scholar] [CrossRef]
- Georgescu, M.-I.; Ionescu, R.T.; Miron, A.-I.; Savencu, O.; Ristea, N.-C.; Verga, N.; Khan, F.S. Multimodal Multi-Head Convolutional Attention with Various Kernel Sizes for Medical Image Super-Resolution. In Proceedings of the 2023 IEEE/CVF Winter Conference on Applications of Computer Vision (WACV), Waikoloa, HI, USA, 2–7 January 2023; pp. 2194–2204. [Google Scholar] [CrossRef]
- Georgescu, M.-I.; Ionescu, R.T.; Miron, A.I. Diversity-Promoting Ensemble for Medical Image Segmentation. In Proceedings of the 38th ACM/SIGAPP Symposium on Applied Computing, Tallinn, Estonia, 27–31 March 2023; Association for Computing Machinery: New York, NY, USA, 2023; pp. 599–606. [Google Scholar]
- Faruqi, F.-A.; Loprinzi, C.-L.; Ruddy, K.-J.; Couch, F.; Staff, N.; Olson, J.-E. Long-Term Neurotoxicity in Women with Breast Cancer. J. Clin. Oncol. 2019, 37, e23089. [Google Scholar] [CrossRef]
- Nilsson, G.; Holmberg, L.; Garmo, H.; Terent, A.; Blomqvist, C. Increased Incidence of Stroke in Women with Breast Cancer. Eur. J. Cancer 2005, 41, 423–429. [Google Scholar] [CrossRef]
- Somerset, W.; Stout, S.C.; Miller, A.H.; Musselman, D. Breast Cancer and Depression. Oncology 2004, 18, 1021–1034; discussion 1035–1036, 1047–1048. [Google Scholar] [PubMed]
- De Fatima, G.G.M.; de Godoy, L.M.P.; Barufi, S.; de Godoy, J.M.P. Pain in Breast Cancer Treatment: Aggravating Factors and Coping Mechanisms. Int. J. Breast Cancer 2014, 2014, 832164. [Google Scholar] [CrossRef]
- Brown, T.; Cruickshank, S.; Noblet, M. Specialist Breast Care Nurses for Support of Women with Breast Cancer. Cochrane Database Syst. Rev. 2021, 2021, CD005634. [Google Scholar] [CrossRef]
- Teodorescu, C.O.D.; Șandru, F.; Charkaoui, A.; Teodorescu, A.; Popa, A.R.; Miron, A.-I. The Dynamic Changes in the Pattern of Liver Function Tests in Pregnant Obese Women. Exp. Ther. Med. 2021, 22, 986. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.C.; de Azambuja, E. Improving Quality of Life after Breast Cancer: Dealing with Symptoms. Maturitas 2011, 70, 343–348. [Google Scholar] [CrossRef]
- Petca, A.; Miron, B.C.; Pacu, I.; Dumitrașcu, M.C.; Mehedințu, C.; Șandru, F.; Petca, R.-C.; Rotar, I.C. HELLP Syndrome-Holistic Insight into Pathophysiology. Medicina 2022, 58, 326. [Google Scholar] [CrossRef] [PubMed]
- Liscu, H.D.; Liscu, B.R.; Mitre, R.; Anghel, I.V.; Antone-Iordache, I.L.; Balan, A.; Coniac, S.; Miron, A.I.; Halcu, G. Medicina|Free Full-Text. The Conditioning of Adjuvant Chemotherapy for Stage II and III Rectal Cancer Determined by Postoperative Pathological Characteristics in Romania. Available online: https://www.mdpi.com/1648-9144/59/7/1224 (accessed on 18 December 2023).
- Sardari, D.; Verga, N. Calculation of Externally Applied Electric Field Intensity for Disruption of Cancer Cell Proliferation. Electromagn. Biol. Med. 2010, 29, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.; Jhaveri, K.; Patel, D.; Gewirtz, A.; Seidman, A.; Kemeny, N. Hepatic Arterial Infusion and Systemic Chemotherapy for Breast Cancer Liver Metastases. Breast J. 2013, 19, 96–99. [Google Scholar] [CrossRef]
- Baseline Quality of Life as a Prognostic Indicator of Survival: A Meta-Analysis of Individual Patient Data from EORTC Clinical Trials—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19695956/ (accessed on 24 January 2024).
- Pinto, B.M.; Maruyama, N.C. Exercise in the Rehabilitation of Breast Cancer Survivors. Psychooncology 1999, 8, 191–206. [Google Scholar] [CrossRef]
- Winick, L.; Robbins, G.F. The Post-Mastectomy Rehabilitation Group Program. Structure, Procedure, and Population Demography. Am. J. Surg. 1976, 132, 599–602. [Google Scholar] [CrossRef]
- Suesada, M.M.; de Andrade Carvalho, H.; de Albuquerque, A.L.P.; Salge, J.M.; Stuart, S.R.; Takagaki, T.Y. Impact of Thoracic Radiotherapy on Respiratory Function and Exercise Capacity in Patients with Breast Cancer. J. Bras. Pneumol. 2018, 44, 469–476. [Google Scholar] [CrossRef]
- The Effect of Immediate Breast Reconstruction with Becker-25 Prosthesis on the Preservation of Proper Body Posture in Patients after Mastectomy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20510569/ (accessed on 24 January 2024).
- dos Santos, D.E.; Rett, M.T.; Mendonça, A.C.R.; Bezerra, T.S.; de Santana, J.M.; Silva Júnior, W.M. da Efeito da radioterapia na função pulmonar e na fadiga de mulheres em tratamento para o câncer de mama. Fisioter. Pesqui. 2013, 20, 50–55. [Google Scholar] [CrossRef]
- Cardiac Toxicity from Systemic Cancer Therapy: A Comprehensive Review—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20728696/ (accessed on 24 January 2024).
- Ionescu (Miron), A.-I.; Atasiei, D.-I.; Ionescu, R.-T.; Ultimescu, F.; Barnonschi, A.-A.; Anghel, A.-V.; Anghel, C.-A.; Antone-Iordache, I.-L.; Mitre, R.; Bobolocu, A.M.; et al. Prediction of Subclinical and Clinical Multiple Organ Failure Dysfunction in Breast Cancer Patients—A Review Using AI Tools. Cancers 2024, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, K.S.; Han, K.; Kim, H.W. A Psychological Intervention Programme for Patients with Breast Cancer under Chemotherapy and at a High Risk of Depression: A Randomised Clinical Trial. J. Clin. Nurs. 2018, 27, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.A.; Agostinetto, E.; Perachino, M.; Del Mastro, L.; de Azambuja, E.; Vaz-Luis, I.; Partridge, A.H.; Lambertini, M. Evidence-Based Approaches for the Management of Side-Effects of Adjuvant Endocrine Therapy in Patients with Breast Cancer. Lancet Oncol. 2021, 22, e303–e313. [Google Scholar] [CrossRef]
- Sharma, N.; Purkayastha, A. Factors Affecting Quality of Life in Breast Cancer Patients: A Descriptive and Cross-Sectional Study with Review of Literature. J. Midlife Health 2017, 8, 75–83. [Google Scholar] [CrossRef]
- Chao, T.-C.; Chen, D.-R.; Chao, T.-Y.; Chen, S.-C.; Yeh, D.-C.; Wang, H.-C.; Huang, W.-T.; Rau, K.-M.; Chang, K.-J.; Yang, T.-L.; et al. Quality of Life Assessment in Taiwanese Patients with Bone Metastases from Breast Cancer Receiving Zoledronic Acid. Anticancer. Res. 2013, 33, 5543–5547. [Google Scholar]
- Guinan, E.M.; Devenney, K.; Quinn, C.; Sheill, G.; Eochagáin, C.M.; Kennedy, M.J.; McDermott, R.; Balding, L. Associations Among Physical Activity, Skeletal Related Events, and Patient Reported Outcomes in Patients with Bone Metastases. Semin. Oncol. Nurs. 2022, 38, 151274. [Google Scholar] [CrossRef] [PubMed]




| Items | N | Median | Mean | 95% Confidence Interval Mean | Std. Deviation | N (%) Score < 33.33% | N (%) Score > 66.66% | |
|---|---|---|---|---|---|---|---|---|
| QLQ-C30 | ||||||||
| Functional scales | ||||||||
| Physical functioning | 607 | 80.000 | 73.465 | 71.824–75.106 | 20.589 | 38 (6.26%) | 445 (73.31%) | |
| Role functioning | 607 | 83.333 | 74.602 | 72.394–76.810 | 27.696 | 113 (18.61%) | 473 (77.92%) | |
| Emotional functional | 607 | 83.333 | 77.732 | 75.969–79.495 | 22.115 | 52 (8.56%) | 488 (80.39%) | |
| Cognitive functioning | 607 | 83.333 | 82.345 | 80.676–84.013 | 20.932 | 37 (6.09%) | 536 (88.30%) | |
| Social functioning | 607 | 100.000 | 89.154 | 87.419–90.890 | 21.770 | 49 (8.07%) | 546 (89.95%) | |
| Symptoms scales | ||||||||
| Fatigue | 607 | 22.222 | 28.263 | 26.322–30.204 | 24.351 | 440 (72.48%) | 84 (13.83%) | |
| Nausea and vomiting | 607 | 0.000 | 10.983 | 9.164–12.802 | 22.823 | 584 (96.21%) | 14 (2.3%) | |
| Pain | 607 | 16.667 | 23.833 | 21.751–25.915 | 26.123 | 477 (78.58%) | 78 (12.85%) | |
| Dyspnea | 607 | 0.000 | 11.148 | 9.351–12.944 | 22.541 | 556 (91.59%) | 51 (8.4%) | |
| Insomnia | 607 | 33.333 | 32.070 | 29.700–34.440 | 29.732 | 431 (71%) | 176 (28.99%) | |
| Appetite loss | 607 | 0.000 | 10.983 | 9.164–12.802 | 22.823 | 576 (94.89%) | 61 (10.04%) | |
| Constipation | 607 | 0.000 | 12.850 | 11.058–14.643 | 22.487 | 551 (90.77%) | 56 (9.22%) | |
| Diarrhea | 607 | 0.000 | 4.448 | 3.325–5.571 | 14.084 | 590 (97.19%) | 17(2.8%) | |
| Financial difficulties | 607 | 0.000 | 19.110 | 16.999–21.221 | 26.482 | 516 (85%) | 91 (14.99%) | |
| Global health status/QoL | 607 | 75.000 | 72.186 | 70.698–73.673 | 18.664 | 33 (5.4%) | 476 (78.41%) | |
| QLQ-C30 summary score | 607 | 85.385 | 82.179 | 81.030–83.327 | 14.409 | 4 (0.65%) | 521 (85.83%) | |
| EORTC QLQ BR-45 | ||||||||
| Functional scales | ||||||||
| Body image | 607 | 100.000 | 83.114 | 81.135–85.092 | 24.824 | 56 (9.22%) | 501 (82.53%) | |
| Sexual functioning * | 607 | 100.000 | 92.724 | 91.291–94.157 | 17.981 | 30 (4.9%) | 568 (93.57%) | |
| Sexual enjoyment * | 88 | 33.333 | 43.939 | 38.634–49.245 | 17.981 | 59 (67.04%) | 29 (32.95%) | |
| Future perspective | 607 | 66.667 | 56.013 | 53.287–58.739 | 34.202 | 275 (45.30%) | 332 (54.69%) | |
| Breast satisfaction * | 539 | 0.000 | 16.759 | 14.800–18.719 | 23.161 | 486 (90.16%) | 37 (6.86%) | |
| Symptoms scales | ||||||||
| Systemic therapy side effect | 607 | 14.286 | 18.655 | 17.388–19.923 | 15.900 | 525 (86.49%) | 4 (0.65%) | |
| Breast Symptoms | 607 | 8.3333 | 11.093 | 10.039–12.146 | 13.217 | 583 (96.04%) | 2 (0.32%) | |
| Arm symptoms | 607 | 11.111 | 18.964 | 17.327–20.601 | 20.535 | 504 (83.03%) | 36 (5.93%) | |
| Upset by hair loss | 199 | 33.333 | 28.978 | 24.503–33.453 | 32.011 | 145 (72.86%) | 54 (27.13%) | |
| Endocrine therapy symptoms | 607 | 20.000 | 21.087 | 19.853–22.321 | 15.479 | 502 (82.70%) | 5 (0.82%) | |
| Skin mucosis symptoms | 607 | 11.111 | 14.488 | 13.402–15.574 | 13.625 | 568 (93.57%) | 1 (0.16%) | |
| Endocrine sexual symptoms | 607 | 0.000 | 9.253 | 7.791–10.715 | 18.341 | 561 (92.42%) | 21 (3.45%) | |
| Variables | PF2 Mean (SD) | RF2 Mean (SD) | EF Mean (SD) | CF Mean (SD) | SF Mean (SD) | QL2 Mean (SD) | QLQ-C30 SS Mean (SD) |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <50 years (N = 67) | 81.99 (15.99) | 76.36 (28.74) | 74.75 (20.61) | 84.08 (21.79) | 88.06 (23.17) | 73.75 (18.13) | 85.31 (10.59) |
| ≥50 years (N = 540) | 72.40 (20.85) | 74.38 (27.58) | 78.10 (22.28) | 82.130 (20.83) | 89.29 (21.60) | 71.99 (18.73) | 81.79 (14.77) |
| p-value | <0.01 | 0.437 | 0.084 | 0.273 | 0.681 | 0.523 | 0.170 |
| Menopausal status | |||||||
| Premenopausal (N = 62) | 81.613 (16.538) | 76.61 (29.01) | 74.32 (21.30) | 83.60 (21.65) | 87.09 (24.59) | 72.44 (18.94) | 84.99 (11.25) |
| Menopausal (N = 545) | 72.53 (20.81) | 74.37 (27.56) | 78.11 (22.19) | 82.20 (20.89) | 89.38 (21.43) | 72.15 (18.64) | 81.85 (14.70) |
| p-value | <0.01 | 0.390 | 0.084 | 0.463 | 0.681 | 0.938 | 0.220 |
| Cancer staging | |||||||
| Stage A = 0, I, II (N = 338) | 76.11 (19.28) | 78.69 (25.83) | 79.14 (22.39) | 83.08 (21.03) | 90.43 (20.63) | 73.81 (17.64) | 83.88 (14.11) |
| Stage B = III, IV (N = 269) | 70.13 (21.69) | 69.45 (29.10) | 75.96 (21.66) | 81.41 (20.80) | 87.54 (23.05) | 70.13 (19.71) | 80.04 (14.51) |
| p-value | <0.01 | <0.01 | 0.013 | 0.180 | 0.074 | 0.034 | <0.01 |
| Type of surgery | |||||||
| Mastectomy (N = 411) | 73.26 (20.45) | 74.16 (28.22) | 77.63 (22.32) | 82.48 (21.33) | 89.05 (22.52) | 72.66 (17.80) | 82.21 (14.29) |
| Conservative breast surgery (N = 127) | 78.11 (18.87) | 82.80 (21.91) | 81.43 (20.73) | 83.33 (19.13) | 91.33 (17.79) | 76.24 (18.00) | 85.24 (12.75) |
| p-value | 0.021 | 0.005 | 0.051 | 0.923 | 0.676 | 0.079 | 0.032 |
| Variables | FA Mean (SD) | NV Mean (SD) | PA Mean (SD) | DY Mean (SD) | SL Mean (SD) | AP Mean (SD) | CO Mean (SD) | DI Mean (SD) | FI Mean (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| <50 years (N = 67) | 24.54 (21.75) | 3.23 (8.32) | 17.66 (21.68) | 6.46 (16.65) | 26.86 (29.72) | 4.47 (15.23) | 10.44 (21.87) | 2.48 (10.56) | 16.41 (27.44) |
| ≥50 years (N = 540) | 28.72 (24.63) | 5.64 (15.07) | 24.59 (26.53) | 11.72 (23.11) | 32.71 (29.69) | 11.79 (23.48) | 13.14 (22.56) | 4.69 (14.45) | 19.44 (26.36) |
| p-value | 0.280 | 0.519 | 0.064 | 0.076 | 0.112 | 0.008 | 0.208 | 0.209 | 0.203 |
| Menopausal status | |||||||||
| Premenopausal (N = 62) | 24.73 (22.26) | 4.03 (10.31) | 17.47 (21.85) | 5.91 (15.41) | 27.95 (29.68) | 5.37 (16.18) | 10.21 (21.41) | 2.68 (10.96) | 15.05(26.08) |
| Menopausal (N = 545) | 28.66 (24.56) | 5.53 (14.90) | 24.55 (26.48) | 11.74 (23.14) | 32.53 (29.72) | 11.62 (23.38) | 13.15 (22.60) | 4.64 (14.391) | 19.57 (26.51) |
| p-value | 0.300 | 0.767 | 0.061 | 0.068 | 0.233 | 0.034 | 0.218 | 0.284 | 0.124 |
| Cancer staging | |||||||||
| Stage A = 0, I, II (N = 338) | 24.91 (23.11) | 5.27 (14.09) | 21.54 (24.93) | 9.76 (21.94) | 30.27 (29.28) | 9.36 (21.35) | 12.03 (22.82) | 3.84 (13.15) | 17.35 (25.44) |
| Stage B = III, IV (N = 269) | 32.46 (25.24) | 5.51 (15.01) | 26.70 (27.32) | 12.88 (23.197) | 34.32 (30.18) | 13.01 (24.43) | 13.87 (22.05) | 5.20 (15.16) | 21.31 (27.62) |
| p-value | <0.001 | 0.864 | 0.020 | 0.027 | 0.109 | 0.038 | 0.106 | 0.181 | 0.073 |
| Type of surgery | |||||||||
| Mastectomy (N = 411) | 27.98 (23.98) | 4.58 (12.80) | 23.56 (26.12) | 11.51 (22.86) | 31.95 (29.37) | 10.38 (22.09) | 13.05 (22.56) | 4.78 (14.58) | 21.00 (27.03) |
| Conservative breast surgery (N = 127) | 23.97 (22.46) | 5.11 (14.47) | 20.21 (22.28) | 8.13 (19.57) | 29.39 (29.87) | 8.66 (20.66) | 11.28 (21.50) | 2.10 (9.151) | 13.64 (24.61) |
| p-value | 0.105 | 0.944 | 0.402 | 0.104 | 0.349 | 0.406 | 0.405 | 0.058 | 0.002 |
| Variables | Body Image Mean (SD) | Future Perspective Mean (SD) | Sexual Functioning Mean (SD) | Sexual Enjoyment Mean (SD) | Breast Satisfaction Mean (SD) |
|---|---|---|---|---|---|
| Age | |||||
| <50 years (N = 67) | 75.62 (29.49) | 55.22 (33.61) | 71.14 (26.20) | 48.64 (28.96) | 11.29 (18.16) |
| ≥50 years (N = 540) | 84.04 (24.05) | 56.11 (34.30) | 95.40 (14.64) | 40.52 (21.41) | 17.43 (23.63) |
| p-value | 0.022 | 0.768 | <0.001 | 0.155 | 0.060 |
| Menopausal status | |||||
| Premenopausal (N= 62) | 76.21 (28.66) | 55.37 (34.64) | 73.11 (26.54) | 47.91 (29.25) | 11.01 (17.77) |
| Menopausal (N= 545) | 83.89 (24.25) | 56.08 (34.18) | 94.95 (15.24) | 41.66 (22.24) | 17.42 (23.63) |
| p-value | 0.028 | 0.842 | <0.001 | 0.263 | 0.057 |
| Cancer staging | |||||
| Stage A = 0, I, II (N = 338) | 83.70 (25.01) | 58.87 (33.50) | 92.55 (18.23) | 41.49 (24.08) | 16.61 (23.87) |
| Stage B = III, IV (N = 269) | 82.37 (24.61) | 52.41 (34.78) | 92.93 (17.68) | 47.00 (26.17) | 16.98 (22.06) |
| p-value | 0.372 | 0.020 | 0.861 | 0.311 | 0.492 |
| Type of surgery | |||||
| Mastectomy (N = 411) | 80.47 (26.53) | 55.96 (34.27) | 93.59 (16.77) | 46.29 (27.02) | 17.56 (23.49) |
| Conservative breast surgery (N = 127) | 89.56 (18.78) | 59.84 (33.15) | 89.23 (21.57) | 37.33 (20.00) | 13.88 (21.98) |
| p-value | 0.001 | 0.271 | 0.017 | 0.198 | 0.072 |
| Variables | SYS Mean (SD) | HU Mean (SD) | ARM Mean (SD) | BR Mean (SD) | ET Mean (SD) | SM Mean (SD) | ES Mean (SD) |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <50 years (N = 67) | 19.82 (14.22) | 20.83 (25.65) | 21.72 (23.08) | 15.17 (12.96) | 20.19 (15.18) | 10.28 (10.64) | 21.02 (25.92) |
| ≥50 years (N = 540) | 18.51 (16.10) | 30.09 (32.69) | 18.62 (20.19) | 10.58 (13.17) | 21.19 (15.52) | 15.01 (13.86) | 7.79 (16.63) |
| p-value | 0.235 | 0.243 | 0.371 | <0.001 | 0.556 | 0.007 | <0.001 |
| Menopausal status | |||||||
| Premenopausal (N = 62) | 20.20 (13.86) | 25.00 (29.89) | 21.14 (22.91) | 14.65 (13.04) | 20.26 (14.10) | 9.94 (10.06) | 20.16 (26.29) |
| Menopausal (N = 545) | 18.48 (16.11) | 29.52 (32.33) | 18.71 (20.25) | 10.68 (13.18) | 21.18 (15.63) | 15.00 (13.885) | 8.01 (16.79) |
| p-value | 0.145 | 0.565 | 0.523 | 0.005 | 0.798 | 0.007 | <0.001 |
| Cancer staging | |||||||
| Stage A = 0, I, II (N = 338) | 17.78 (16.12) | 30.03 (32.83) | 17.52 (20.04) | 10.45 (12.85) | 20.04 (15.31) | 13.28 (12.82) | 9.36 (18.51) |
| Stage B = III, IV (N = 269) | 19.75 (15.57) | 27.89 (31.27) | 20.77 (21.03) | 11.89 (13.63) | 22.39 (15.61) | 16.00 (14.44) | 9.10 (18.16) |
| p-value | 0.030 | 0.701 | 0.028 | 0.148 | 0.046 | 0.015 | 0.765 |
| Type of surgery | |||||||
| Mastectomy (N = 411) | 18.41 (15.86) | 28.43 (31.56) | 20.33 (21.31) | 11.13 (13.33) | 21.21 (15.57) | 14.93 (13.27) | 9.16 (19.22) |
| Conservative breast surgery (N = 127) | 17.81 (15.84) | 31.66 (32.86) | 15.04 (18.28) | 9.18 (11.91) | 11.94 (12.79) | 11.94 (12.79) | 9.97 (17.22) |
| p-value | 0.587 | 0.588 | 0.009 | 0.110 | 0.260 | 0.006 | 0.212 |
| Items | Heart Failure | Kidney Failure | Neurologic Dysfunction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | p-Value | N | Mean (SD) | p-Value | N | Mean (SD) | p-Value | ||||
| QLQ-C30 | ||||||||||||
| Functional scales | ||||||||||||
| Physical functioning | HF− | 356 | 77.80 (17.50) | <0.001 | KF− | 500 | 74.62 (19.52) | 0.018 | N− | 502 | 73.49 (20.75) | 0.795 |
| HF+ | 251 | 67.30 (22.96) | KF+ | 107 | 68.03 (24.34) | N+ | 105 | 73.33 (19.87) | ||||
| Role functioning | HF− | 356 | 77.80 (17.50) | 0.086 | KF− | 500 | 75.10 (27.34) | 0.415 | N− | 502 | 75.06 (27.67) | 0.298 |
| HF+ | 251 | 71.91 (29.52) | KF+ | 107 | 72.27 (29.31) | N+ | 105 | 72.38 (27.81) | ||||
| Emotional functioning | HF− | 356 | 76.42 (22.70) | 0.098 | KF− | 500 | 77.16 (22.10) | 0.074 | N− | 502 | 78.25 (22.099) | 0.061 |
| HF+ | 251 | 79.58 (21.16) | KF+ | 107 | 80.37 (22.08) | N+ | 105 | 75.23 (21.13) | ||||
| Cognitive functioning | HF− | 356 | 82.72 (21.49) | 0.318 | KF− | 500 | 82.56 (21.23) | 0.268 | N− | 502 | 82.30 (21.44) | 0.567 |
| HF+ | 251 | 81.80 (20.13) | KF+ | 107 | 81.30 (19.52) | N+ | 105 | 82.54 (18.40) | ||||
| Social functioning | HF− | 356 | 89.37 (21.86) | 0.615 | KF− | 500 | 88.83 (2.09) | 0.701 | N− | 502 | 88.87 (21.98) | 0.459 |
| HF+ | 251 | 88.84 (21.74) | KF+ | 107 | 90.65 (20.24) | N+ | 105 | 90.47 (20.78) | ||||
| Symptom scales | ||||||||||||
| Fatigue | HF− | 356 | 26.96 (24.06) | 0.112 | KF− | 500 | 22.75 (23.75) | 0.487 | N− | 502 | 28.02 (24.61) | 0.402 |
| HF+ | 251 | 30.10 (24.68) | KF+ | 107 | 30.63 (26.95) | N+ | 105 | 29.14 (23.11) | ||||
| Nausea and vomiting | HF− | 356 | 5.47 (14.65) | 0.840 | KF− | 500 | 5.20 (14.10) | 0.617 | N− | 502 | 5.37 (14.51) | 0.826 |
| HF+ | 251 | 5.24 (14.30) | KF+ | 107 | 6.23 (16.27) | N+ | 105 | 5.39 (14.52) | ||||
| Pain | HF− | 356 | 22.09 (25.76) | 0.034 | KF− | 500 | 23.56 (25.98) | 0.602 | N− | 502 | 22.61 (26.23) | 0.002 |
| HF+ | 251 | 26.29 (26.48) | KF+ | 107 | 25.07 (26.83) | N+ | 105 | 29.68 (24.89) | ||||
| Dyspnea | HF− | 356 | 9.83 (20.32) | 0.190 | KF− | 500 | 10.60 (21.44) | 0.590 | N− | 502 | 11.28 (22.40) | 0.642 |
| HF+ | 251 | 13.01 (25.27) | KF+ | 107 | 13.70 (27.07) | N+ | 105 | 10.47 (23.25) | ||||
| Insomnia | HF− | 356 | 29.213 (28.22) | 0.010 | KF− | 500 | 31.20 (29.84) | 0.091 | N− | 502 | 31.93 (30.08) | 0.671 |
| HF+ | 251 | 36.12 (31.35) | KF+ | 107 | 36.13 (29.00) | N+ | 105 | 32.69 (28.11) | ||||
| Appetite loss | HF− | 356 | 9.83 (21.84) | 0.129 | KF− | 500 | 10.40 (21.99) | 0.282 | N− | 502 | 10.95 (22.81) | 0.955 |
| HF+ | 251 | 12.61 (24.13) | KF+ | 107 | 13.70 (26.28) | N+ | 105 | 11.11 (22.95) | ||||
| Constipation | HF− | 356 | 11.61 (21.43) | 0.112 | KF− | 500 | 12.80 (22.54) | 0.792 | N− | 502 | 12.94 (22.84) | 0.920 |
| HF+ | 251 | 14.60 (23.82) | KF+ | 107 | 13.04 (22.31) | N+ | 105 | 12.38 (20.80) | ||||
| Diarheea | HF− | 356 | 3.83 (13.27) | 0.118 | KF− | 500 | 4.53 (14.05) | 0.497 | N− | 502 | 4.51 (14.34) | 1.000 |
| HF+ | 251 | 5.31 (15.14) | KF+ | 107 | 4.05 (14.26) | N+ | 105 | 4.12 (12.82) | ||||
| Financial difficulties | HF− | 356 | 19.00 (26.43) | 0.898 | KF− | 500 | 19.06 (26.29) | 0.935 | N− | 502 | 19.65 (27.43) | 0.748 |
| HF+ | 251 | 19.25 (26.60) | KF+ | 107 | 19.31 (27.48) | N+ | 105 | 19.65 (27.43) | ||||
| Global health status/QoL | HF− | 356 | 73.47 (18.71) | 0.024 | KF− | 500 | 72.78 (18.47) | 0.057 | N− | 502 | 72.44 (18.68) | 0.510 |
| HF+ | 251 | 70.35 (18.46) | KF+ | 107 | 69.39 (19.35) | N+ | 105 | 70.95 (18.60) | ||||
| QLQ-C30 summary score | HF− | 356 | 83.38 (13.80) | 0.010 | KF− | 500 | 82.48 (14.10) | 0.481 | N− | 502 | 82.33 (14.58) | 0.323 |
| HF+ | 251 | 80.47 (15.08) | KF+ | 107 | 80.77 (15.76) | N+ | 105 | 81.43 (13.55) | ||||
| EORTC QLQ-BR45 | ||||||||||||
| functional scales | ||||||||||||
| Body image | HF− | 356 | 81.22 (26.43) | 0.053 | KF− | 500 | 82.00 (25.46) | 0.007 | N− | 502 | 82.55 (25.42) | 0.309 |
| HF+ | 251 | 85.79 (22.12) | KF+ | 107 | 88.31 (20.91) | N+ | 105 | 85.79 (21.64) | ||||
| Sexual functioning | HF− | 356 | 88.15 (21.54) | <0.001 | KF− | 500 | 91.53 (19.15) | <0.001 | N− | 502 | 92.72 (18.01) | 0.962 |
| HF+ | 251 | 99.20 (7.25) | KF+ | 107 | 98.28 (9.13) | N+ | 105 | 92.69 (17.89) | ||||
| Sexual enjoyment | HF− | 85 | 44.70 (24.96) | 0.117 | KF− | 84 | 43.65 (25.33) | 0.491 | N− | 72 | 44.44 (26.24) | 0.860 |
| HF+ | 3 | 22.22 (19.24) | KF+ | 4 | 50.00 (19.24) | N+ | 16 | 41.66 (19.24) | ||||
| Future perspective | HF− | 356 | 53.18 (34.29) | 0.015 | KF- | 500 | 55.40 (34.47) | 0.329 | N− | 502 | 55.77 (33.97) | 0.732 |
| HF+ | 251 | 60.02 (33.73) | KF+ | 107 | 58.87 (32.88) | N+ | 105 | 57.11 (35.42) | ||||
| Breast satisfaction | HF− | 356 | 15.23 (21.86) | 0.078 | KF− | 500 | 16.55 (23.63) | 0.254 | N− | 445 | 16.81 (23.41) | 0.900 |
| HF+ | 251 | 19.08 (24.87) | KF+ | 107 | 17.81 (20.61) | N+ | 94 | 16.48 (22.06) | ||||
| Symptoms scales | ||||||||||||
| Systemic therapy side effects | HF− | 356 | 18.70 (16.69) | 0.539 | KF− | 500 | 18.93 (16.08) | 0.347 | N− | 502 | 18.69 (16.19) | 0.688 |
| HF+ | 251 | 18.59 (14.72) | KF+ | 107 | 17.35 (15.00) | N+ | 105 | 18.45 (14.49) | ||||
| Breast symptoms | HF− | 356 | 12.36 (13.50) | <0.001 | KF− | 500 | 11.43 (13.74) | 0.517 | N− | 502 | 11.33 (13.52) | 0.528 |
| HF+ | 251 | 9.29 (12.60) | KF+ | 107 | 9.50 (10.33) | N+ | 105 | 9.92 (11.61) | ||||
| Arm symptoms | HF− | 356 | 19.85 (21.14) | 0.240 | KF− | 500 | 19.37 (20.75) | 0.278 | N− | 502 | 19.21 (20.93) | 0.850 |
| HF+ | 251 | 17.70 (19.60) | KF+ | 107 | 17.03 (19.44) | N+ | 105 | 17.77 (18.52) | ||||
| Upset by hair loss | HF− | 114 | 30.11 (32.57) | 0.576 | KF− | 166 | 29.51 (32.70) | 0.747 | N− | 171 | 29.04 (31.84) | 0.891 |
| HF+ | 85 | 27.45 (31.36) | KF+ | 33 | 26.26 (28.57) | N+ | 28 | 28.57 (33.59) | ||||
| Endocrine therapy symptoms | HF− | 356 | 20.27 (15.70) | 0.054 | KF− | 500 | 21.32 (15.65) | 0.507 | N− | 502 | 20.70 (15.78) | 0.063 |
| HF+ | 251 | 22.24 (15.10) | KF+ | 107 | 20.00 (14.65) | N+ | 105 | 22.92 (13.84) | ||||
| Skin mucosis symptoms | HF− | 356 | 13.73 (13.37) | 0.065 | KF− | 500 | 14.50 (13.70) | 0.928 | N− | 502 | 14.48 (14.21) | 0.230 |
| HF+ | 251 | 15.56 (13.92) | KF+ | 107 | 14.43 (13.30) | N+ | 105 | 14.49 (10.408) | ||||
| Endocrine sexual symptoms | HF− | 356 | 11.84 (20.95) | <0.001 | KF− | 500 | 10.08 (19.09) | 0.009 | N− N+ | 502 | 9.04 (18.21) | 0.256 |
| HF+ | 251 | 5.57 (12.99) | KF+ | 107 | 5.37 (13.74) | 105 | 10.23 (18.99) | |||||
| Items | Liver Failure | Respiratory Failure | GI Dysfunction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | p-Value | N | Mean (SD) | p-Value | N | Mean (SD) | p-Value | |||||
| QLQ-C30 | |||||||||||||
| Functional scales | |||||||||||||
| Physical functioning | LF− | 526 | 73.96 (20.23) | 0.248 | RF− | 515 | 74.82 (19.72) | <0.001 | GI− | 550 | 73.45 (20.77) | 0.827 | |
| LF+ | 81 | 70.20 (22.63) | RF+ | 92 | 65.87 (23.59) | GI+ | 57 | 73.56 (18.89) | |||||
| Role functioning | LF− | 526 | 75.57 (27.12) | 0.054 | RF− | 515 | 76.37 (26.66) | <0.001 | GI− | 550 | 74.78 (27.50) | 0.698 | |
| LF+ | 81 | 68.31 (30.57) | RF+ | 92 | 64.67 (31.23) | GI+ | 57 | 72.80 (29.65) | |||||
| Emotional functioning | LF− | 526 | 78.24 (22.03) | 0.105 | RF− | 515 | 78.35 (21.74) | 0.137 | GI− | 550 | 77.87 (22.11) | 0.536 | |
| LF+ | 81 | 74.38 (22.50) | RF+ | 92 | 74.27 (23.91) | GI+ | 57 | 76.31 (22.26) | |||||
| Cognitive functioning | LF− | 526 | 83.08 (20.59) | 0.018 | RF− | 515 | 83.07 (20.63) | 0.037 | GI− | 550 | 82.18 (21.04) | 0.496 | |
| LF+ | 81 | 77.57 (22.54) | RF+ | 92 | 78.26 (22.20) | GI+ | 57 | 83.91 (19.91) | |||||
| Social functioning | LF− | 526 | 89.63 (21.32) | 0.209 | RF− | 515 | 90.64 (20.38) | <0.001 | GI− | 550 | 89.09 (21.97) | 0.883 | |
| LF+ | 81 | 86.00 (24.36) | RF+ | 92 | 80.79 (26.94) | GI+ | 57 | 89.76 (19.85) | |||||
| Symptoms scales | |||||||||||||
| Fatigue | LF− | 526 | 27.60 (23.99) | 0.147 | RF− | 515 | 26.88 (23.35) | 0.006 | GI− | 550 | 28.50 (24.41) | 0.449 | |
| LF+ | 81 | 32.51 (26.33) | RF+ | 92 | 35.99 (28.21) | GI+ | 57 | 25.92 (23.88) | |||||
| Nausea and vomiting | LF− | 526 | 5.29 (14.80) | 0.086 | RF− | 515 | 5.08 (13.89) | 0.427 | GI− | 550 | 5.36 (14.43) | 0.829 | |
| LF+ | 81 | 5.96 (12.44) | RF+ | 92 | 7.06 (17.51) | GI+ | 57 | 5.55 (15.21) | |||||
| Pain | LF− | 526 | 23.38 (25.53) | 0.542 | RF− | 515 | 22.33 (25.07) | 0.003 | GI− | 550 | 23.81 (26.08) | 0.997 | |
| LF+ | 81 | 26.74 (29.66) | RF+ | 92 | 32.24 (30.14) | GI+ | 57 | 23.97 (26.72) | |||||
| Dyspnea | LF− | 526 | 10.83 (22.22) | 0.477 | RF− | 515 | 9.83 (21.16) | 0.001 | GI− | 550 | 11.39 (22.83) | 0.437 | |
| LF+ | 81 | 13.16 (24.54) | RF+ | 92 | 18.47 (28.11) | GI+ | 57 | 8.77 (19.44) | |||||
| Insomnia | LF− | 526 | 31.36 (29.35) | 0.168 | RF− | 515 | 30.48 (29.04) | 0.003 | GI− | 550 | 32.42 (29.79) | 0.382 | |
| LF+ | 81 | 36.62 (31.88) | RF+ | 92 | 40.94 (32.06) | GI+ | 57 | 28.65 (29.16) | |||||
| Appetite loss | LF− | 526 | 10.83 (22.79) | 0.660 | RF− | 515 | 10.03 (22.02) | 0.007 | GI− | 550 | 10.97 (23.00) | 0.682 | |
| LF+ | 81 | 11.93 (23.15) | RF+ | 92 | 16.30 (26.37) | GI+ | 57 | 11.11 (21.20) | |||||
| Constipation | LF− | 526 | 12.42(22.10) | 0.264 | RF− | 515 | 11.97 (21.73) | 0.033 | GI− | 550 | 12.35 (21.77) | 0.264 | |
| LF+ | 81 | 15.63 (24.76) | RF+ | 92 | 17.75 (25.89) | GI+ | 57 | 17.54 (28.24) | |||||
| Diarheea | LF− | 526 | 4.30 (13.58) | 0.792 | RF− | 515 | 4.33 (13.65) | 0.852 | GI− | 550 | 4.18 (13.67) | 0.153 | |
| LF+ | 81 | 5.35 (17.04) | RF+ | 92 | 5.07 (16.34) | GI+ | 57 | 7.01 (17.52) | |||||
| Financial difficulties | LF− | 526 | 18.88 (26.55) | 0.459 | RF− | 515 | 18.18 (26.04) | 0.035 | GI− | 550 | 19.09 (26.63) | 0.752 | |
| LF+ | 81 | 20.57 (26.12) | RF+ | 92 | 24.27 (28.43) | GI+ | 57 | 19.29 (25.15) | |||||
| Global health status/QoL | LF− | 526 | 72.33 (18.30) | 0.860 | RF− | 515 | 73.33 (17.60) | 0.002 | GI− | 550 | 72.22 (18.74) | 0.693 | |
| LF+ | 81 | 71.19 (20.91) | RF+ | 92 | 65.76 (22.79) | GI+ | 57 | 71.78 (18.00) | |||||
| QLQ-C30 summary score | LF− | 526 | 82.65 (14.22) | 0.045 | RF− | 515 | 83.25 (13.79) | <0.001 | GI− | 550 | 82.18 (14.50) | 0.781 | |
| LF+ | 81 | 79.11 (15.26) | RF+ | 92 | 76.15 (16.28) | GI+ | 57 | 82.14 (13.54) | |||||
| EORTC QLQ-BR45 | |||||||||||||
| Functional scales | |||||||||||||
| Body image | LF− | 526 | 83.39 (17.52) | 0.648 | RF− | 515 | 83.57 (24.61) | 0.320 | GI− | 550 | 82.98 (25.11) | 0.843 | |
| LF+ | 81 | 81.27 (27.27) | RF+ | 92 | 80.52 (25.95) | GI+ | 57 | 84.35 (21.99) | |||||
| Sexual functioning | LF− | 526 | 92.55 (18.22) | 0.575 | RF− | 515 | 92.00 (18.63) | 0.005 | GI− | 550 | 92.69 (17.95) | 0.624 | |
| LF+ | 81 | 93.82 (16.33) | RF+ | 92 | 96.73 (13.13) | GI+ | 57 | 92.98 (18.35) | |||||
| Sexual enjoyment | LF− | 77 | 42.85 (24.69) | 0.402 | RF− | 82 | 44.30 (25.68) | 0.628 | GI− | 80 | 45.41 (25.01) | 0.083 | |
| LF+ | 11 | 51.51 (27.34) | RF+ | 6 | 38.88 (13.60) | GI+ | 8 | 29.16 (21.36) | |||||
| Future perspective | LF− | 526 | 56.71 (33.87) | 0.223 | RF− | 515 | 57.54 (33.79) | 0.011 | GI− | 550 | 56.24 (34.09) | 0.600 | |
| LF+ | 81 | 51.44 (36.15) | RF+ | 92 | 47.46 (35.37) | GI+ | 57 | 53.80 (35.49) | |||||
| Breast Satisfaction | LF− | 469 | 16.98 (23.48) | 0.751 | RF− | 480 | 16.52 (23.02) | 0.591 | GI− | 487 | 16.87 (23.32) | 0.780 | |
| LF+ | 70 | 15.23 (20.99) | RF+ | 59 | 18.64 (24.38) | GI+ | 52 | 15.70 (21.74) | |||||
| Symptoms scales | |||||||||||||
| Systemic therapy side effects | LF− | 526 | 18.58 (15.96) | 0.582 | RF− | 515 | 18.07 (15.50) | 0.063 | GI+ | 550 | 18.61 (15.75) | 0.889 | |
| LF+ | 81 | 19.10 (15.54) | RF+ | 92 | 21.89 (17.67) | GI− | 57 | 19.04 (17.35) | |||||
| Breast symptoms | LF− | 526 | 11.15 (13.411) | 0.950 | RF− | 515 | 10.55 (12.53) | 0.062 | GI+ | 550 | 11.06 (13.00) | 0.966 | |
| LF+ | 81 | 10.70 (11.94) | RF+ | 92 | 14.13 (16.28) | GI− | 57 | 11.40 (15.23) | |||||
| Arm symptoms | LF− | 526 | 19.24 (20.66) | 0.377 | RF− | 515 | 18.87 (20.39) | 0.971 | GI+ | 550 | 19.07 (20.64) | 0.783 | |
| LF+ | 81 | 17.14 (19.72) | RF+ | 92 | 19.44 (21.39) | GI− | 57 | 17.93 (19.55) | |||||
| Upset by hair loss | LF− | 172 | 29.07 (32.36) | 0.974 | RF− | 164 | 28.25 (32.10) | 0.421 | GI+ | 179 | 29.05 (32.58) | 0.854 | |
| LF+ | 27 | 28.39 (30.24) | RF+ | 35 | 32.38 (31.81) | GI− | 20 | 28.33 (27.09) | |||||
| Endocrine therapy symptoms | LF− | 526 | 20.84 (15.61) | 0.192 | RF− | 515 | 20.88 (15.27) | 0.506 | GI+ | 550 | 21.33 (15.38) | 0.126 | |
| LF+ | 81 | 22.67 (14.55) | RF+ | 92 | 22.21 (16.63) | GI− | 57 | 18.71 (16.30) | |||||
| Skin mucosis symptoms | LF− | 526 | 14.18 (13.53) | 0.131 | RF− | 515 | 13.98 (13.00) | 0.104 | GI+ | 550 | 14.23 (13.52) | 0.178 | |
| LF+ | 81 | 16.46 (14.12) | RF+ | 92 | 17.33 (16.46) | GI− | 57 | 16.95 (14.48) | |||||
| Endocrine sexual symptoms | LF− | 526 | 77.78 (17.52) | 0.300 | RF− | 515 | 10.12 (19.23) | 0.008 | GI+ | 550 | 9.71 (18.71) | 0.064 | |
| LF+ | 81 | 9.41 (18.38) | RF+ | 92 | 4.34 (11.01) | GI− | 57 | 4.82 (13.54) | |||||
| Items | M1 Non-Oss | M1 Oss | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| QLQ-C30 | |||||||
| Functional scales | |||||||
| Physical functioning | 49 | 70.47 | 20.95 | 74 | 58.28 | 25.21 | 0.006 |
| Role functioning | 49 | 68.02 | 28.83 | 74 | 58.10 | 32.43 | 0.090 |
| Emotional functional | 49 | 75.34 | 26.84 | 74 | 71.62 | 21.00 | 0.130 |
| Cognitive functioning | 49 | 81.63 | 21.03 | 74 | 77.47 | 23.47 | 0.369 |
| Social functioning | 49 | 86.39 | 24.45 | 74 | 79.95 | 28.13 | 0.115 |
| Symptoms scales | |||||||
| Fatigue | 49 | 32.42 | 27.76 | 74 | 42.34 | 28.05 | 0.053 |
| Nausea and vomiting | 49 | 4.76 | 12.72 | 74 | 11.48 | 22.22 | 0.071 |
| Pain | 49 | 24.83 | 27.66 | 74 | 41.44 | 30.62 | 0.002 |
| Dyspnea | 49 | 16.32 | 26.46 | 74 | 16.21 | 28.26 | 0.865 |
| Insomnia | 49 | 39.45 | 32.39 | 74 | 41.44 | 32.55 | 0.746 |
| Appetite loss | 49 | 12.92 | 24.35 | 74 | 21.62 | 29.42 | 0.088 |
| Constipation | 49 | 12.24 | 21.18 | 74 | 20.27 | 26.92 | 0.100 |
| Diarrhea | 49 | 3.40 | 12.25 | 74 | 8.10 | 20.50 | 0.185 |
| Financial difficulties | 49 | 23.81 | 28.05 | 74 | 20.72 | 26.28 | 0.541 |
| Global health status/ QoL | 49 | 70.91 | 18.79 | 74 | 59.12 | 21.77 | 0.004 |
| QLQ-C30 summary score | 49 | 79.65 | 16.34 | 74 | 72.50 | 16.93 | 0.019 |
| EORTC QLQ BR-45 | |||||||
| Functional scales | |||||||
| Body image | 49 | 82.48 | 26.80 | 74 | 86.48 | 23.47 | 0.549 |
| Sexual functioning | 49 | 96.59 | 14.01 | 74 | 94.14 | 16.87 | 0.287 |
| Sexual enjoyment | 3 | 33.33 | 0.00 | 9 | 44.44 | 23.57 | N |
| Future perspective | 49 | 48.29 | 36.68 | 74 | 44.59 | 34.13 | 0.588 |
| Breast satisfaction | 37 | 13.06 | 16.72 | 43 | 20.15 | 25.08 | 0.261 |
| Symptoms scales | |||||||
| Systemic therapy side effects | 49 | 17.88 | 16.13 | 74 | 22.52 | 17.41 | 0.116 |
| Breast symptoms | 49 | 11.73 | 13.91 | 74 | 13.85 | 16.10 | 0.390 |
| Arm symptoms | 49 | 19.04 | 21.27 | 74 | 22.52 | 21.12 | 0.305 |
| Upset by hair loss | 12 | 41.66 | 20.71 | 24 | 27.77 | 34.98 | 0.075 |
| Endocrine therapy symptoms | 49 | 17.95 | 13.90 | 74 | 26.26 | 16.44 | 0.002 |
| Skin mucosis symptoms | 49 | 13.94 | 13.32 | 74 | 19.52 | 16.71 | 0.052 |
| Endocrine sexual symptoms | 49 | 3.57 | 9.62 | 74 | 7.09 | 15.88 | 0.372 |
| Variable | Age ≥ 50 | Stage B | RF+ | HF+ | LF+ | KF+ | N+ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | Adj. R2 | p Value | |
| QL2 | −0.006 | 0.889 | −0.073 | 0.075 | −0.126 | 0.002 | −0.060 | 0.155 | 0.011 | 0.795 | −0.053 | 0.201 | −0.029 | 0.201 | 0.024 | 0.003 |
| PF2 | −0.084 | 0.036 | −0.122 | 0.002 | −0.107 | 0.007 | −0.206 | <0.001 | −0.025 | 0.521 | −0.063 | 0.114 | −0.004 | 0.917 | 0.097 | <0.001 |
| RF2 | −0.006 | 0.880 | −0.138 | <0.001 | −0.110 | 0.008 | −0.065 | 0.122 | −0.055 | 0.172 | −0.017 | 0.669 | −0.032 | 0.424 | 0.041 | <0.001 |
| EF | 0.023 | 0.587 | −0.056 | 0.174 | −0.054 | 0.195 | 0.062 | 0.148 | −0.048 | 0.240 | 0.044 | 0.293 | −0.045 | 0.262 | 0.009 | 0.083 |
| CF | −0.025 | 0.556 | −0.021 | 0.613 | −0.065 | 0.121 | −0.005 | 0.904 | −0.077 | 0.064 | −0.012 | 0.779 | 0.006 | 0.877 | 0.003 | 0.283 |
| SF | 0.018 | 0.663 | −0.035 | 0.395 | −0.153 | <0.001 | −0.010 | 0.813 | −0.031 | 0.446 | 0.040 | 0.335 | 0.035 | 0.390 | 0.020 | 0.007 |
| FA | 0.044 | 0.294 | 0.133 | 0.001 | 0.098 | 0.018 | 0.037 | 0.382 | 0.038 | 0.354 | 0.024 | 0.556 | 0.018 | 0.650 | 0.031 | <0.001 |
| PA | 0.072 | 0.085 | 0.046 | 0.062 | 0.110 | 0.008 | 0.051 | 0.225 | 0.016 | 0.686 | −0.0006 | 0.988 | 0.100 | 0.013 | 0.032 | <0.001 |
| DY | 0.057 | 0.178 | 0.047 | 0.254 | 0.122 | 0.004 | 0.038 | 0.375 | 0.009 | 0.823 | 0.031 | 0.457 | −0.015 | 0.709 | 0.018 | 0.014 |
| SL | 0.032 | 0.449 | 0.044 | 0.280 | 0.102 | 0.014 | 0.089 | 0.035 | 0.036 | 0.384 | 0.036 | 0.387 | 0.009 | 0.826 | 0.021 | 0.006 |
| AP | 0.090 | 0.033 | 0.068 | 0.101 | 0.081 | 0.053 | 0.023 | 0.596 | −0.005 | 0.899 | 0.034 | 0.417 | 0.003 | 0.950 | 0.014 | 0.029 |
| CO | 0.024 | 0.573 | 0.024 | 0.563 | 0.078 | 0.063 | 0.054 | 0.204 | 0.033 | 0.417 | −0.014 | 0.731 | −0.012 | 0.770 | 0.003 | 0.277 |
| DI | 0.044 | 0.303 | 0.048 | 0.251 | 0.003 | 0.947 | 0.044 | 0.301 | 0.021 | 0.620 | −0.031 | 0.462 | −0.012 | 0.776 | −0.004 | 0.694 |
| FI | 0.040 | 0.350 | 0.064 | 0.126 | 0.071 | 0.091 | −0.012 | 0.777 | 0.005 | 0.895 | −0.006 | 0.885 | −0.048 | 0.239 | 0.003 | 0.283 |
| SS | −0.057 | 0.168 | −0.102 | 0.012 | −0.142 | <0.001 | −0.068 | 0.107 | −0.048 | 0.232 | −0.015 | 0.708 | −0.020 | 0.620 | 0.044 | <0.001 |
| Variable | Age ≥ 50 | Stage B | RF+ | HF+ | LF+ | KF+ | N+ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | ꞵ | Sig. | Adj. R2 | p Value | |
| BI | 0.081 | 0.055 | −0.015 | 0.716 | −0.049 | 0.240 | 0.060 | 0.159 | −0.026 | 0.530 | 0.079 | 0.056 | 0.057 | 0.160 | 0.016 | 0.018 |
| FU | −0.025 | 0.549 | −0.076 | 0.065 | −0.096 | 0.021 | 0.110 | 0.010 | −0.034 | 0.403 | 0.029 | 0.483 | 0.022 | 0.591 | 0.020 | 0.008 |
| SX | 0.364 | <0.001 | 0.011 | 0.757 | 0.062 | 0.096 | 0.192 | <0.001 | 0.006 | 0.863 | 0.050 | 0.176 | 0.009 | 0.803 | 0.216 | <0.001 |
| SE | −0.206 | 0.073 | 0.152 | 0.188 | −0.077 | 0.506 | −0.151 | 0.176 | 0.084 | 0.448 | 0.100 | 0.367 | −0.003 | 0.978 | 0.015 | 0.321 |
| BS | 0.065 | 0.145 | 0.011 | 0.793 | 0.023 | 0.601 | 0.064 | 0.158 | −0.030 | 0.493 | 0.002 | 0.968 | −0.005 | 0.903 | 0.001 | 0.479 |
| SYS | −0.021 | 0.613 | 0.047 | 0.255 | 0.080 | 0.057 | 0.003 | 0.953 | −0.004 | 0.917 | −0.039 | 0.349 | −0.011 | 0.794 | 0.000 | 0.411 |
| HU | 0.123 | 0.104 | −0.045 | 0.536 | 0.068 | 0.367 | −0.073 | 0.337 | −0.021 | 0.777 | −0.047 | 0.527 | −0.020 | 0.782 | 0.014 | 0.755 |
| ARM | −0.029 | 0.487 | 0.082 | 0.048 | 0.007 | 0.866 | −0.038 | 0.371 | −0.041 | 0.320 | −0.033 | 0.425 | −0.030 | 0.457 | 0.002 | 0.313 |
| BR | −0.082 | 0.052 | 0.036 | 0.381 | 0.108 | 0.009 | −0.096 | 0.023 | −0.028 | 0.493 | −0.030 | 0.464 | −0.048 | 0.235 | 0.025 | 0.002 |
| ET | 0.014 | 0.743 | 0.071 | 0.089 | 0.007 | 0.874 | 0.067 | 0.118 | 0.032 | 0.434 | −0.049 | 0.243 | 0.051 | 0.207 | 0.004 | 0.219 |
| SM | 0.106 | 0.012 | 0.089 | 0.030 | 0.060 | 0.149 | 0.037 | 0.383 | 0.039 | 0.338 | −0.031 | 0.458 | −0.002 | 0.954 | 0.019 | 0.010 |
| ES | −0.189 | <0.001 | 0.005 | 0.906 | −0.098 | 0.016 | −0.101 | 0.015 | −0.003 | 0.949 | −0.046 | 0.251 | 0.020 | 0.608 | 0.065 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, A.-I.; Anghel, A.-V.; Antone-Iordache, I.-L.; Atasiei, D.-I.; Anghel, C.-A.; Barnonschi, A.-A.; Bobolocu, A.-M.; Verga, C.; Șandru, F.; Lișcu, H.-D. Assessing the Impact of Organ Failure and Metastases on Quality of Life in Breast Cancer Patients: A Prospective Study Based on Utilizing EORTC QLQ-C30 and EORTC QLQ-BR45 Questionnaires in Romania. J. Pers. Med. 2024, 14, 214. https://doi.org/10.3390/jpm14020214
Ionescu A-I, Anghel A-V, Antone-Iordache I-L, Atasiei D-I, Anghel C-A, Barnonschi A-A, Bobolocu A-M, Verga C, Șandru F, Lișcu H-D. Assessing the Impact of Organ Failure and Metastases on Quality of Life in Breast Cancer Patients: A Prospective Study Based on Utilizing EORTC QLQ-C30 and EORTC QLQ-BR45 Questionnaires in Romania. Journal of Personalized Medicine. 2024; 14(2):214. https://doi.org/10.3390/jpm14020214
Chicago/Turabian StyleIonescu (Miron), Andreea-Iuliana, Alexandra-Valentina Anghel, Ionuț-Lucian Antone-Iordache, Dimitrie-Ionuț Atasiei, Cătălin-Alexandru Anghel, Andrei-Alexandru Barnonschi, Alexandra-Maria Bobolocu, Catinca Verga, Florica Șandru, and Horia-Dan Lișcu. 2024. "Assessing the Impact of Organ Failure and Metastases on Quality of Life in Breast Cancer Patients: A Prospective Study Based on Utilizing EORTC QLQ-C30 and EORTC QLQ-BR45 Questionnaires in Romania" Journal of Personalized Medicine 14, no. 2: 214. https://doi.org/10.3390/jpm14020214
APA StyleIonescu, A. -I., Anghel, A. -V., Antone-Iordache, I. -L., Atasiei, D. -I., Anghel, C. -A., Barnonschi, A. -A., Bobolocu, A. -M., Verga, C., Șandru, F., & Lișcu, H. -D. (2024). Assessing the Impact of Organ Failure and Metastases on Quality of Life in Breast Cancer Patients: A Prospective Study Based on Utilizing EORTC QLQ-C30 and EORTC QLQ-BR45 Questionnaires in Romania. Journal of Personalized Medicine, 14(2), 214. https://doi.org/10.3390/jpm14020214