Putative Drone Copulation Factors Regulating Honey Bee (Apis mellifera) Queen Reproduction and Health: A Review
Abstract
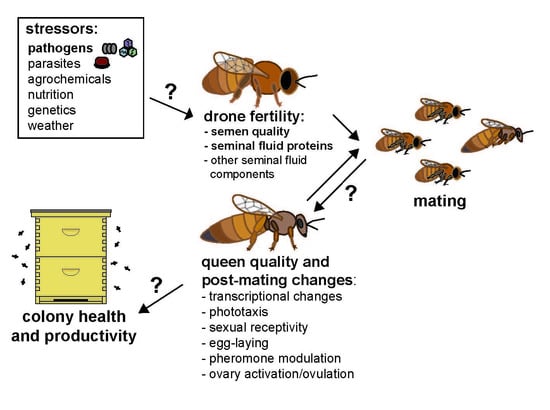
:1. Introduction/Background
2. Honey Bee Queen Post-Mating Changes
2.1. Behavioral Post-Mating Changes
2.2. Physiological Post-Mating Changes
2.3. Molecular Post-Mating Changes
3. Copulation Factors Influencing Queen Post-Mating Changes and Reproduction
3.1. Effects of Drone Number and Insemination Volume on Post-Mating Changes
3.2. Effects of Insemination Fluid Composition on Post-Mating Changes
4. Seminal Fluid Proteins and Their Potential Roles in Queen Post-Mating Changes and Health
4.1. Seminal Fluid Functions in Drosophila and Other Insects
4.2. Identification of Honey Bee Seminal Fluid Proteins and Their Potential Roles in Queen Post-Mating Changes
4.3. Roles of Honey Bee Seminal Fluid Proteins in Pathogen Defense
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Cornman, R.S.; Tarpy, D.R.; Chen, Y.; Jeffreys, L.; Lopez, D.; Pettis, J.S.; Evans, J.D. Pathogen webs in collapsing honey bee colonies. PLoS ONE 2012, 7, e43562. [Google Scholar] [CrossRef] [PubMed]
- Ravoet, J.; Maharramov, J.; Meeus, I.; De Smet, L.; Wenseleers, T.; Smagghe, G.; de Graaf, D.C. Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS ONE 2013, 8, e72443. [Google Scholar] [CrossRef] [PubMed]
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.M.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; et al. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apic. Res. 2017, 56, 328–340. [Google Scholar] [CrossRef]
- Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; Underwood, R.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Ellis, J.D.; Evans, J.D.; Pettis, J. Colony losses, managed colony population decline, and Colony Collapse Disorder in the United States. J. Apic. Res. 2010, 49, 134–136. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; Vanengelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef]
- Cavigli, I.; Daughenbaugh, K.F.; Martin, M.; Lerch, M.; Banner, K.; Garcia, E.; Brutscher, L.M.; Flenniken, M.L. Pathogen prevalence and abundance in honey bee colonies involved in almond pollination. Apidologie 2015, 251–266. [Google Scholar] [CrossRef]
- Glenny, W.; Cavigli, I.; Daughenbaugh, K.F.; Radford, R.; Kegley, S.E.; Flenniken, M.L. Honey bee (Apis mellifera) colony health and pathogen composition in migratory beekeeping operations involved in California almond pollination. PLoS ONE 2017, 12, 1–24. [Google Scholar] [CrossRef]
- Seitz, N.; Traynor, K.S.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Ellis, J.D.; Rose, R.; Tarpy, D.R.; Sagili, R.R.; Caron, D.M.; et al. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apic. Res. 2015, 54, 292–304. [Google Scholar] [CrossRef]
- Delaney, D.A.; Keller, J.J.; Caren, J.R.; Tarpy, D.R. The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.). Apidologie 2011, 42, 1–13. [Google Scholar] [CrossRef]
- Rangel, J.; Keller, J.J.; Tarpy, D.R. The effects of honey bee (Apis mellifera L.) queen reproductive potential on colony growth. Insectes Soc. 2013, 60, 65–73. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987; ISBN 0-674-07409-2. [Google Scholar]
- Baer, B. Sexual selection in Apis bees. Apidologie 2005, 36, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, J.W.; Harden, S.; Spooner-Hart, R.; Anderson, D.L.; Wheen, G. Effects of age, season and genetics on semen and sperm production in Apis mellifera drones. Apidologie 2011, 42, 29–38. [Google Scholar] [CrossRef]
- Rhodes, J. Drone Honey Bees-Rearing and Maintenance; NSW Agriculture: Orange, Australia, 2002. [Google Scholar]
- Czekońska, K.; Chuda-Mickiewicz, B. The ability of honey bee drones to ejaculate. J. Apic. Sci. 2015, 59, 127–133. [Google Scholar] [CrossRef]
- Czekonska, K.; Chuda-Mickiewicz, B.; Samborski, J. Quality of honeybee drones reared in colonies with limited and unlimited access to pollen. Apidologie 2015, 46, 1–9. [Google Scholar] [CrossRef]
- Moritz, R.F.A. The Instrumental Insemination of the Queen Bee; APIMONDIA: Bucharest, Romania, 1989. [Google Scholar]
- Ellis, J.; Lawrence, J.C.; Koeniger, N.; Koeniger, G. Mating Biology of Honey Bees (Apis mellifera); Wicwas Press: Kalamazoo, MI, USA, 2015; ISBN 978-1878075383. [Google Scholar]
- Kraus, F.B.; Neumann, P.; Moritz, R.F.A. Genetic variance of mating frequency in the honeybee (Apis mellifera L.). Insectes Soc. 2005, 52, 1–5. [Google Scholar] [CrossRef]
- Withrow, J.M.; Tarpy, D.R. Cryptic “ royal” subfamilies in honey bee (Apis mellifera) colonies. PLoS ONE 2018, 13, e019912. [Google Scholar] [CrossRef]
- Woyke, J.; Ruttner, F. An Anatomical Study of the Mating Process in the Honeybee. Bee World 1958, 39, 3–18. [Google Scholar] [CrossRef]
- Woyke, J. Natural and Artificial Insemination of Queen Honeybees. Bee World 1962, 43, 21–25. [Google Scholar] [CrossRef]
- Schluns, H.; Moritz, R.F.A.; Neumann, P.; Kryger, P.; Koeniger, G. Multiple nuptial flights, sperm transfer and the evolution of extreme polyandry in honeybee queens. Anim. Behav. 2005, 70, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Koeniger, N.; Koeniger, G. Reproductive isolation among species of the genus Apis To cite this version. Apidologie 2000, 31, 313–339. [Google Scholar] [CrossRef]
- Baer, B.; Collins, J.; Maalaps, K.; den Boer, S.P.A. Sperm use economy of honeybee (Apis mellifera) queens. Ecol. Evol. 2016, 6, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Queen quality and the impact of honey bee diseases on queen health: Potential for interactions between two major threats to colony health. Insects 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Tarpy, D.R.; Keller, J.J.; Caren, J.R.; Delaney, D.A. Assessing the Mating ‘Health’ of Commercial Honey Bee Queens. J. Econ. Entomol. 2012, 105, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Woyke, J. Correlations between the Age at Which Honeybee Brood Was Grafted, Characteristics of the Resultant Queens, and Results of Insemination. J. Apic. Res. 1971, 10, 45–55. [Google Scholar] [CrossRef]
- Tibor; Szabo, I.; Mills, P.F.; Heikel, D.T. Effects of Honeybee Queen Weight and Air Temperature on the Initiation of Oviposition. J. Apic. Res. 1987, 26, 73–78. [Google Scholar] [CrossRef]
- Collins, A.M.; Pettis, J.S. Correlation of queen size and spermathecal contents and effects of miticide exposure during development. Apidologie 2013, 44, 351–356. [Google Scholar] [CrossRef]
- Szabo, T.I.; Townsend, G.F. Behavioural Studies on Queen Introduction in the Honeybee 1. Effect of the Age of Workers (From a Colony with a Laying Queen) on their Behaviour towards an Introduced Virgin Queen. J. Apic. Res. 1974, 13, 19–25. [Google Scholar] [CrossRef]
- Gregorc, A.; Smodiš Škerl, M.I. Characteristics of honey bee (Apis mellifera carnica, Pollman 1879) queens reared in Slovenian commercial breeding stations. J. Apic. Sci. 2015, 59, 5–12. [Google Scholar] [CrossRef]
- Kahya, Y.; Gençer, H.V.; Woyke, J. Weight at emergence of honey bee (Apis mellifera caucasica) queens and its effect on live weights at the pre and post mating periods. J. Apic. Res. 2008, 47, 118–125. [Google Scholar] [CrossRef]
- Gilley, D.C.; Tarpy, D.R.; Land, B.B. Effect of queen quality on interactions between workers and dueling queens in honeybee (Apis mellifera L.) colonies. Behav. Ecol. Sociobiol. 2003, 55, 190–196. [Google Scholar] [CrossRef]
- Akyol, E.; Yeninar, H.; Kaftanoglu, O. Live Weight of Queen Honey Bees (Apis mellifera L.) Predicts Reproductive Characteristics (Bal Arilarinda (Apis mellifera L.) Bazi Üreme Özelliklerinin Belirlenmesinde Ana Ari Aǧirliǧinin Kullanimi). J. Kansas Entomol. Soc. 2018, 81, 92–100. [Google Scholar] [CrossRef]
- Al-Lawati, H.; Kamp, G.; Bienefeld, K. Characteristics of the spermathecal contents of old and young honeybee queens. J. Insect Physiol. 2009, 55, 116–121. [Google Scholar] [CrossRef]
- Lodesani, M.; Balduzzi, D.; Galli, A. Functional characterisation of semen in honeybee queen (A. m. ligustica S.) spermatheca and efficiency of the diluted semen technique in instrumental insemination. Ital. J. Anim. Sci. 2004, 3, 385–392. [Google Scholar] [CrossRef]
- Mackensen, O. Relation of Semen Volume to Success in Artificial Insemination of Queen Honey Bees. J. Econ. Entomol. 1964, 57, 581–583. [Google Scholar] [CrossRef]
- Rangel, J.; Böröczky, K.; Schal, C.; Tarpy, D.R. Honey Bee (Apis mellifera) Queen Reproductive Potential Affects Queen Mandibular Gland Pheromone Composition and Worker Retinue Response. PLoS ONE 2016, 11, e0156027. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Hatch, S.; Fletcher, D.J.C. The influence of queen age and quality during queen replacement in honeybee colonies. Anim. Behav. 2000, 59, 97–101. [Google Scholar] [CrossRef]
- Hatch, S.; Tarpy, D.R.; Fletcher, D.J.C. Worker regulation of emergency queen rearing in honey bee colonies and the resultant variation in queen quality. Insectes Soc. 1999, 46, 372–377. [Google Scholar] [CrossRef]
- Haarmann, T.; Spivak, M.; Weaver, D.; Weaver, B.; Glenn, T. Effects of Fluvalinate and Coumaphos on Queen Honey Bees (Hymenoptera: Apidae) in Two Commercial Queen Rearing Operations. J. Econ. Entomol. 2002, 95, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sandrock, C.; Tanadini, M.; Tanadini, L.G.; Fauser-Misslin, A.; Potts, S.G.; Neumann, P. Impact of chronic neonicotinoid exposure on honeybee colony performance and queen supersedure. PLoS ONE 2014, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu-Smart, J.; Spivak, M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.J.; Tarpy, D.R.; Grozinger, C.M. Effects of insemination quantity on honey bee queen physiology. PLoS ONE 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.J.; Schal, C.; Tarpy, D.R.; Grozinger, C.M. Effects of Instrumental Insemination and Insemination Quantity on Dufour’s Gland Chemical Profiles and Vitellogenin Expression in Honey Bee Queens (Apis mellifera). J. Chem. Ecol. 2011, 37, 1027–1036. [Google Scholar] [CrossRef]
- Kocher, S.D.; Richard, F.; Tarpy, D.R.; Grozinger, C.M.; State, N.C. Queen reproductive state modulates pheromone production and queen-worker interactions in honeybees. Behav. Ecol. 2009, 20, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niño, E.L.; Malka, O.; Hefetz, A.; Teal, P.; Hayes, J.; Grozinger, C.M. Effects of honey bee (Apis mellifera L.) queen insemination volume on worker behavior and physiology. J. Insect Physiol. 2012, 58, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Niño, E.L.; Malka, O.; Hefetz, A.; Tarpy, D.R.; Grozinger, C.M. Chemical profiles of two pheromone glands are differentially regulated by distinct mating factors in honey bee queens (Apis mellifera L.). PLoS ONE 2013, 8, e78637. [Google Scholar] [CrossRef] [PubMed]
- Peso, M.; Niño, E.L.; Grozinger, C.M.; Barron, A.B. Effect of honey bee queen mating condition on worker ovary activation. Insectes Soc. 2013, 60, 123–133. [Google Scholar] [CrossRef]
- Keeling, C.I.; Slessor, K.N.; Higo, H.A.; Winston, M.L. New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc. Natl. Acad. Sci. USA 2003, 100, 4486–4491. [Google Scholar] [CrossRef]
- Slessor, K.N.; Kaminski, L.A.; King, G.G.S.; Winston, M.L. Semiochemicals of the honeybee queen mandibular glands. J. Chem. Ecol. 1990, 16, 851–860. [Google Scholar] [CrossRef]
- Pankiw, T.; Winston, M.L.; Plettner, E.; Slessor, K.N.; Pettis, J.S.; Taylor, O.R. Mandibular gland components of European and Africanized honey bee queens (Apis mellifera L.). J. Chem. Ecol. 1996, 22, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.D.; Hartfelder, K. The initial stages of oogenesis and their relation to differential fertility in the honey bee (Apis mellifera) castes. Arthropod Struct. Dev. 2004, 33, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Kocher, S.D.; Richard, F.-J.; Tarpy, D.R.; Grozinger, C.M. Genomic analysis of post-mating changes in the honey bee queen (Apis mellifera). BMC Genom. 2008, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Niño, E.L.; Tarpy, D.R.; Grozinger, C.M. Differential effects of insemination volume and substance on reproductive changes in honey bee queens (Apis mellifera L.). Insect Mol. Biol. 2013, 22, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.E.; Evison, S.E.F.; Baer, B.; Hughes, W.O.H. The cost of promiscuity: Sexual transmission of Nosema microsporidian parasites in polyandrous honey bees. Sci. Rep. 2015, 5, 10982. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Fries, I. Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J. Invertebr. Pathol. 2008, 98, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.; Meixner, M.D.; Kryger, P. Deformed wing virus can be transmitted during natural mating in honey bees and infect the queens. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Schröder, M.; Bienefeld, K.; Genersch, E. Detection of viral sequences in semen of honeybees (Apis mellifera): Evidence for vertical transmission of viruses through drones. J. Invertebr. Pathol. 2006, 92, 93–96. [Google Scholar] [CrossRef]
- Johnson, J.N.; Hardgrave, E.; Gill, C.; Moore, D. Absence of consistent diel rhythmicity in mated honey bee queen behavior. J. Insect Physiol. 2010, 56, 761–773. [Google Scholar] [CrossRef]
- Al-Qarni, A.; Phelan, P.; Smith, B.H.; Cobey, S.W. The influence of mating type and oviposition period on mandibular pheromone levels in Apis mellifera L. honeybee queens. Saudi J. Biol. Sci. 2005, 12, 39–47. [Google Scholar]
- Katzav Gozansky, T.; Soroker, V.; Hefetz, A. The biosynthesis of Dufour’s gland constituents of the honeybee (Apis mellifera) in queens. Invertebr. Neurosci. 1997, 3, 239–243. [Google Scholar] [CrossRef]
- Butler, C.G.; Simpson, J. The Introduction of Virgin and Mated Queens, Directly and in a Simple Cage. Bee World 1956, 37, 105–124. [Google Scholar] [CrossRef]
- Szabo, T.I. Behavioural Studies of Queen Introduction in the Honeybee 6. Multiple Queen Introduction. J. Apic. Res. 1977, 16, 65–83. [Google Scholar] [CrossRef]
- Kocher, S.D.; Tarpy, D.R.; Grozinger, C.M. The effects of mating and instrumental insemination on queen honey bee flight behaviour and gene expression. Insect Mol. Biol. 2010, 19, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettis, J.S.; Westcott, L.C.; Winston, M.L. Balling behaviour in the honey bee in response to exogenous queen mandibular gland pheromone. J. Apic. Res. 1998, 37, 125–131. [Google Scholar] [CrossRef]
- Free, J.B.; Ferguson, A.W.R.; Simpkins, J. Influence of immature queen honeybees (Apis mellifera) on queen rearing and foraging. Physiol. Entomol. 1984, 9, 387–394. [Google Scholar] [CrossRef]
- Smart, M.; Pettis, J.; Rice, N.; Browning, Z.; Spivak, M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE 2016, 11, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Patrício, K.; Cruz-Landim, C. Mating influence in the ovary differentiation in adult queens of Apis mellifera L. (Hymenoptera, Apidae). Braz. J. Biol. 2002, 62, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Shehata, S.M.; Townsend, G.F.; Shuel, R.W. Seasonal Physiological Changes in Queen and Worker Honeybees. J. Apic. Res. 1981, 20, 69–78. [Google Scholar] [CrossRef]
- Slessor, K.N.; Winston, M.L.; Le Conte, Y. Pheromone communication in the honeybee (Apis mellifera L.). J. Chem. Ecol. 2005, 31, 2731–2745. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Sharabash, N.M.; Whitfield, C.W.; Robinson, G.E. Pheromone-mediated gene expression in the honey bee brain. Proc. Natl. Acad. Sci. USA 2003, 100, 14519–14525. [Google Scholar] [CrossRef] [Green Version]
- Villar, G.; Wolfson, M.D.; Hefetz, A.; Grozinger, C.M. Evaluating the Role of Drone-Produced Chemical Signals in Mediating Social Interactions in Honey Bees (Apis mellifera). J. Chem. Ecol. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Slessor, K.N.; Kaminski, L.A.; King, G.G.S.; Borden, J.H.; Winston, M.L. Semiochemical basis of the retinue response to queen honey bees. Nature 1988, 332, 354–356. [Google Scholar] [CrossRef]
- Hoover, S.E.R.; Keeling, C.I.; Winston, M.L.; Slessor, K.N. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 2003, 90, 477–480. [Google Scholar] [CrossRef]
- Winston, M.L.; Slessor, K.N.; Willis, L.G.; Naumann, K.; Higo, H.A.; Wyborn, M.H.; Kaminski, L.A. The influence of queen mandibular pheromones on worker attraction to swarm clusters and inhibition of queen rearing in the honey bee (Apis mellifera L.). Insectes Soc. 1989, 36, 15–27. [Google Scholar] [CrossRef]
- Pankiw, T.; Huang, Z.Y.; Winston, M.L.; Robinson, G.E. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J. Insect Physiol. 1998, 44, 685–692. [Google Scholar] [CrossRef]
- Pettis, J.S.; Winston, M.L.; Collins, A.M. Suppression of queen rearing in European and Africanized honey bees Apis mellifera L. by synthetic queen mandibular gland pheromone. Insectes Soc. 1995, 42, 113–121. [Google Scholar] [CrossRef]
- Higo, H.A.; Colley, S.J.; Winston, M.L.; Slessor, K.N. Effects Of Honey Bee (Apis Mellifera L.) Queen Mandibular Gland Pheromone On Foraging And Brood Rearing. Can. Entomol. 1992, 124, 409–418. [Google Scholar] [CrossRef]
- Fischer, P.; Grozinger, C.M. Pheromonal regulation of starvation resistance in honey bee workers (Apis mellifera). Naturwissenschaften 2008, 95, 723–729. [Google Scholar] [CrossRef]
- Plettner, E.; Otis, G.W.; Wimalaratne, P.D.C.; Winston, M.L.; Slessor, K.N.; Pankiw, T. Species- And Caste-Determined Mandibular Gland Signals In Honeybees (Apis). J. Chem. Ecol. 1997, 23, 363–377. [Google Scholar] [CrossRef]
- Katzav-Gozansky, T.; Soroker, V.; Ibarra, F.; Francke, W.; Hefetz, A. Dufour’s gland secretion of the queen honeybee (Apis mellifera): An egg discriminator pheromone or a queen signal? Behav. Ecol. Sociobiol. 2001, 51, 76–86. [Google Scholar] [CrossRef]
- Katzav-Gozansky, T.; Soroker, V.; Francke, W.; Hefetz, A. Honeybee egg-laying workers mimic a queen signal. Insectes Soc. 2003, 50, 20–23. [Google Scholar] [CrossRef]
- Wossler, T.C.; Crewe, R.M. The releaser effects of the tergal gland secretion of queen honeybees (Apis mellifera). J. Insect Behav. 1999, 12, 343–351. [Google Scholar] [CrossRef]
- Smith, R.-K.; Spivak, M.; Taylor, O.R.; Bennett, C.; Smith, M.L. Maturation of tergal gland alkene profiles in European honey bee queens, Apis mellifera L. J. Chem. Ecol. 1993, 19, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Harano, K.I.; Sasaki, K.; Nagao, T. Depression of brain dopamine and its metabolite after mating in European honeybee (Apis mellifera) queens. Naturwissenschaften 2005, 92, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, S.E.; Giray, T.; Robinson, G.E. Volume Changes in the Mushroom Bodies of Adult Honey Bee Queens. Neurobiol. Learn. Mem. 1995, 63, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.N.; Ing, N.; Rangel, J. Upregulation of antioxidant genes in the spermathecae of honey bee (Apis mellifera) queens after mating. Apidologie 2018, 49, 224–234. [Google Scholar] [CrossRef]
- Manfredini, F.; Brown, M.J.F.; Vergoz, V.; Oldroyd, B.P. RNA-sequencing elucidates the regulation of behavioural transitions associated with the mating process in honey bee queens. BMC Genom. 2015, 16, 563. [Google Scholar] [CrossRef]
- Niño, E.L.; Tarpy, D.R.; Grozinger, C. Genome-wide analysis of brain transcriptional changes in honey bee (Apis mellifera L.) queens exposed to. Insect Mol. Biol. 2011, 20, 387–398. [Google Scholar] [CrossRef]
- Dalton, J.E.; Kacheria, T.S.; Knott, S.R.V.; Lebo, M.S.; Nishitani, A.; Sanders, L.E.; Stirling, E.J.; Winbush, A.; Arbeitman, M.N. Dynamic, mating-induced gene expression changes in female head and brain tissues of Drosophila melanogaster. BMC Genom. 2010, 11. [Google Scholar] [CrossRef]
- Manfredini, F.; Romero, A.E.; Pedroso, I.; Paccanaro, A.; Sumner, S.; Brown, M.J.F. Neurogenomic signatures of successes and failures in life-history transitions in a key insect pollinator. Genome Biol. Evol. 2017, 9, 3059–3072. [Google Scholar] [CrossRef] [PubMed]
- Vergoz, V.; Lim, J.; Duncan, M.; Cabanes, G.; Oldroyd, B.P. Effects of natural mating and CO2 narcosis on biogenic amine receptor gene expression in the ovaries and brain of queen honey bees, Apis mellifera. Insect Mol. Biol. 2012, 21, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.W.; Woodring, J.; Harbo, J.R. Effects of carbon dioxide on levels of biogenic amines in the brains of queenless worker and virgin queen honey bees (Apis mellifera). J. Apic. Res. 1996, 35, 69–78. [Google Scholar] [CrossRef]
- Cobey, S.W.; Tarpy, D.R.; Woyke, J. Standard methods for instrumental insemination of Apis mellifera queens. J. Apic. Res. 2013, 52, 1–18. [Google Scholar] [CrossRef]
- Mackensen, O. Effect of Carbon Dioxide on Initial Oviposition of Artificially Inseminated and Virgin Queen Bees. J. Econ. Entomol. 1947, 40, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Seeley, T.D. Genetic diversity in Honey Bee Colonies Enhances Fitness and Productivity. Science 2015, 317, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Tarpy, D.R. Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeley, T.D.; Tarpy, D.R. Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. Lond. B 2007, 274, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Tarpy, D.R.; Seeley, T.D. Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs monandrous queens. Naturwissenschaften 2006, 93, 195–199. [Google Scholar] [CrossRef]
- Wedell, N. Female receptivity in butterflies and moths. J. Exp. Biol. 2005, 208, 3433–3440. [Google Scholar] [CrossRef] [Green Version]
- Liberti, J.; Görner, J.; Welch, M.; Dosselli, R.; Schiøtt, M.; Ogawa, Y.O.; Castleden, I.; Hemmi, J.M.; Baer-Imhoof, B.; Boomsma, J.J.; et al. Male manipulation of queen visual perception: A novel expression of sexual conflict in the honeybee. 2018. in review. [Google Scholar]
- Niño, E.L. Department of Entomology and Nematology, University of California, Davis, CA, USA. Personal Communication, 2018. [Google Scholar]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect Seminal Fluid Proteins: Identification and Function. Annu. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirot, L.K.; Wong, A.; Chapman, T.; Wolfner, M.F. Sexual conflict and seminal fluid proteins: A dynamic landscape of sexual interactions. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.D.; Yi, X.; MacCoss, M.J.; Swanson, W.J. Proteomics Reveals Novel Drosophila Seminal Fluid Proteins Transferred at Mating. PLoS Biol. 2008, 6, e178. [Google Scholar] [CrossRef] [PubMed]
- Baer, B.; Heazlewood, J.L.; Taylor, N.L.; Eubel, H.; Millar, A.H. The seminal fluid proteome of the honeybee Apis mellifera. Proteomics 2009, 9, 2085–2097. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M.; Caperna, T.J.; Williams, V.; Garrett, W.M.; Evans, J.D. Proteomic analyses of male contributions to honey bee sperm storage and mating. Insect Mol. Biol. 2006, 15, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Colonello, N.A.; Hartfelder, K. She’s my girl—Male accessory gland products and their function in the reproductive biology of social bees. Apidologie 2005, 36, 231–244. [Google Scholar] [CrossRef]
- Pilch, B.; Mann, M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006, 7. [Google Scholar] [CrossRef]
- De Tozetto, S.O.; Gentile Bitondi, M.M.; Dallacqua, R.P.; Paulino Simoes, Z.L. Protein profiles of testes, seminal vesicles and accessory glands of honey bee pupae and their relation to the ecdysteroid titer. Apidologie 2007, 38, 1–11. [Google Scholar] [CrossRef]
- Poiani, A. Complexity of seminal fluid: A review. Behav. Ecol. Sociobiol. 2006, 60, 289–310. [Google Scholar] [CrossRef]
- Wolfner, M.F. Tokens of love: Functions and regulation of Drosophila male accessory gland products. Insect Biochem. Mol. Biol. 1997, 27, 179–192. [Google Scholar] [CrossRef]
- Wolfner, M.F. The gifts that keep on giving: Physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity 2002, 88, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sirot, L.K. Ask not (only) what proteomics can do for behavior, but (also) what behavior can do for proteomes: A comment on Valcu and Kempenaers. Behav. Ecol. 2015, 26, 17. [Google Scholar] [CrossRef]
- Ram, K.R.; Wolfner, M.F. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 2007, 47, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Holman, L. Drosophila melanogaster seminal fluid can protect the sperm of other males. Funct. Ecol. 2009, 23, 180–186. [Google Scholar] [CrossRef]
- Herndon, L.A.; Wolfner, M.F. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl. Acad. Sci. USA 1995, 92, 10114–10118. [Google Scholar] [CrossRef] [PubMed]
- Lung, O.; Kuo, L.; Wolfner, M.F. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J. Insect Physiol. 2001, 47, 617–622. [Google Scholar] [CrossRef]
- Bertram, M.J.; Neubaum, D.M.; Wolfner, M.F. Localization of the Drosophila male accessory gland protein Acp36DE in the mated female suggests a role in sperm storage. Insect Biochem. Mol. Biol. 1996, 26, 971–980. [Google Scholar] [CrossRef]
- Chapman, T. Seminal fluid-mediated fitness traits in Drosophila. Heredity 2001, 87, 511. [Google Scholar] [CrossRef]
- Neubaum, D.M.; Wolfner, M.F. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 1999, 153, 845–857. [Google Scholar]
- Ram, K.R.; Wolfner, M.F. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 2007, 3, 2428–2438. [Google Scholar] [CrossRef]
- Agner, W.I.E.W.; Elley, R.O.J.K.; Ucker, K.A.R.T.; Arper, C.H.J.H. Females Receive A Life-Span Benefit From Male Ejaculates In A Field Cricket. Evolution 2001, 55, 994–1001. [Google Scholar] [CrossRef]
- Fan, Y.; Applebaum, S.W. Drosophila melanogaster sex peptide stimulates juvenile hormone synthesis and depresses sex pheromone production in Helicoverpa armigera. J. Insect Physiol. 1999, 45, 127–133. [Google Scholar] [CrossRef]
- Goenaga, J.; Yamane, T.; Rönn, J.; Arnqvist, G. Within-species divergence in the seminal fluid proteome and its effect on male and female reproduction in a beetle. BMC Evol. Biol. 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hanin, O.; Azrielli, A.; Zakin, V.; Applebaum, S.; Rafaeli, A. Identifcation and differential expression of a sex-peptide receptor in Helicoverpa armigera. Insect Biochem. Mol. Biol. 2011, 41, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Kingan, T.G.; Bodnar, W.M.; Raina, A.K.; Shabanowitz, J.; Hunt, D.F. The loss of female sex pheromone after mating in the corn earworm moth Helicoverpa zea: Identification of a male pheromonostatic peptide. Proc. Natl. Acad. Sci. USA 1995, 92, 5082–5086. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.W.; Baldini, F.; Battaglia, F.; Panico, M.; Dell, A.; Morris, H.R.; Catteruccia, F. Transglutaminase-Mediated Semen Coagulation Controls Sperm Storage in the Malaria Mosquito. PLoS Biol. 2009, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Q. Seminal fluid reduces female longevity and stimulates egg production and sperm trigger oviposition in a moth. J. Insect Physiol. 2011, 57, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Goenaga, J.; Rönn, J.L.; Arnqvist, G. Male seminal fluid substances affect sperm competition success and female reproductive behavior in a seed beetle. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Yamane, T.; Miyatake, T. Reduced female mating receptivity and activation of oviposition in two Callosobruchus species due to injection of biogenic amines. J. Insect Physiol. 2010, 56, 271–276. [Google Scholar] [CrossRef]
- Gillott, C.M. Modulators of Female Reproductive Physiology and Behavior. Annu. Rev. Entomol. 2003, 48, 163–184. [Google Scholar] [CrossRef]
- King, M.; Eubel, H.; Millar, A.H.; Baer, B. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J. Insect Physiol. 2011, 57, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, S.P.A.; Baer, B.; Boomsma, J.J. Seminal Fluid Mediates Ejaculate Competition in Social Insects. Science 2010, 327, 1506–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, B. Bumblebees as model organisms to study male sexual selection in social insects. Behav. Ecol. Sociobiol. 2003, 54, 521–533. [Google Scholar] [CrossRef]
- Yamane, T.; Kimura, Y.; Katsuhara, M.; Miyatake, T. Female mating receptivity inhibited by injection of male-derived extracts in Callosobruchus chinensis. J. Insect Physiol. 2008, 54, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Baer, B.; Armitage, S.A.O.; Boomsma, J.J. Sperm storage induces an immunity cost in ants. Nature 2006, 441, 872–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Findlay, G.D.; Swanson, W.J. Proteomics enhances evolutionary and functional analysis of reproductive proteins. BioEssays 2010, 32, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.L.; Huestis, D.L.; Garcia, C.; Hiromasa, Y.; Wheeler, S.; Noh, S.; Tomich, J.M.; Howard, D.J. Comparative proteomics uncovers the signature of natural selection acting on the ejaculate proteomes of two cricket species isolated by postmating, prezygotic phenotypes. Mol. Biol. Evol. 2011, 28, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Heifetz, Y.; Lung, O.; Frongillo, E.A.; Wolfner, M.F. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 2000, 10, 99–102. [Google Scholar] [CrossRef]
- Grassl, J.; Peng, Y.; Baer-Imhoof, B.; Welch, M.; Millar, A.H.; Baer, B. Infections with the Sexually Transmitted Pathogen Nosema apis Trigger an Immune Response in the Seminal Fluid of Honey Bees (Apis mellifera). J. Proteome Res. 2017, 16, 319–334. [Google Scholar] [CrossRef]
- Baer, B.; Zareie, R.; Paynter, E.; Poland, V.; Millar, A.H. Seminal fluid proteins differ in abundance between genetic lineages of honeybees. J. Proteomics 2012, 75, 5646–5653. [Google Scholar] [CrossRef]
- Kurze, C.; Dosselli, R.; Grassl, J.; Le Conte, Y.; Kryger, P.; Baer, B.; Moritz, R.F.A. Differential proteomics reveals novel insights into Nosema–honey bee interactions. Insect Biochem. Mol. Biol. 2016, 79, 42–49. [Google Scholar] [CrossRef]
- Peng, Y.; Grassl, J.; Millar, A.H.; Baer, B. Seminal fluid of honeybees contains multiple mechanisms to combat infections of the sexually transmitted pathogen Nosema apis. Proc. R. Soc. B Biol. Sci. 2016, 283, 20151785. [Google Scholar] [CrossRef]
- Poland, V.; Eubel, H.; King, M.; Solheim, C.; Harvey Millar, A.; Baer, B. Stored sperm differs from ejaculated sperm by proteome alterations associated with energy metabolism in the honeybee Apis mellifera. Mol. Ecol. 2011, 20, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Zareie, R.; Eubel, H.; Millar, A.H.; Baer, B. Long-term survival of high quality sperm: Insights into the sperm proteome of the honeybee Apis mellifera. J. Proteome Res. 2013, 12, 5180–5188. [Google Scholar] [CrossRef]
- Stürup, M.; Baer-Imhoof, B.; Nash, D.R.; Boomsma, J.J.; Baer, B. When every sperm counts: Factors affecting male fertility in the honeybee Apis mellifera. Behav. Ecol. 2013, 24, 1192–1198. [Google Scholar] [CrossRef]
- Hunter, F.M.; Birkhead, T.R. Sperm viability and sperm competition in insects. Curr. Biol. 2002, 12, 121–123. [Google Scholar] [CrossRef]
- Den Boer, S.P.A.; Boomsma, J.J.; Baer, B. Honey bee males and queens use glandular secretions to enhance sperm viability before and after storage. J. Insect Physiol. 2009, 55, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Baer, B.; Morgan, E.D.; Schmid-Hempel, P. A nonspecific fatty acid within the bumblebee mating plug prevents females from remating. Proc. Natl. Acad. Sci. USA 2001, 98, 3926–3928. [Google Scholar] [CrossRef] [Green Version]
- Baer, B.; Maile, R.; Schmid-Hempel, P.; Morgan, E.D.; Jones, G.R. Chemistry of a mating plug in bumblebees. J. Chem. Ecol. 2000, 26, 1869–1875. [Google Scholar] [CrossRef]
- Sauter, A.; Brown, M.J.F.; Baer, B.; Schmid-Hempel, P. Males of social insects can prevent queens from multiple mating. Proc. R. Soc. B Biol. Sci. 2001, 268, 1449–1454. [Google Scholar] [CrossRef] [Green Version]
- Iovinella, I.; Dani, F.R.; Niccolini, A.; Sagona, S.; Michelucci, E.; Gazzano, A.; Turillazzi, S.; Felicioli, A.; Pelosi, P. Differential expression of odorant-binding proteins in the mandibular glands of the honey bee according to caste and age. J. Proteome Res. 2011, 10, 3439–3449. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Virus and dsRNA-triggered transcriptional responses reveal key components of honey bee antiviral defense. Sci. Rep. 2017, 7, 6448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cornman, R.S.; Lopez, D.; Evans, J.D. Transcriptional response of honey bee larvae infected with the bacterial pathogen Paenibacillus larvae. PLoS ONE 2013, 8, e65424. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, A.J.; Daughenbaugh, K.F.; Fenali, P.; Pizzorno, M.; Flenniken, M.L. Honey Bee and Bumble Bee Antiviral Defense. Viruses 2018, 10, 395. [Google Scholar] [CrossRef]
- Merkling, S.H.; Overheul, G.J.; van Mierlo, J.T.; Arends, D.; Gilissen, C.; van Rij, R.P. The heat shock response restricts virus infection in Drosophila. Sci. Rep. 2015, 5, 12758. [Google Scholar] [CrossRef]
- Knell, R.J.; Webberley, K.M. Sexually transmitted diseases of insects: Distribution, evolution, ecology and host behaviour. Biol. Rev. Camb. Philos. Soc. 2004, 79, 557–581. [Google Scholar] [CrossRef]
- Schmid, M.R.; Brockmann, A.; Pirk, C.W.W.; Stanley, D.W.; Tautz, J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol. 2008, 54, 439–444. [Google Scholar] [CrossRef]
- Rueppell, O.; Aumer, D.; Moritz, R.F. Ties between ageing plasticity and reproductive physiology in honey bees (Apis mellifera) reveal a positive relation between fecundity and longevity as consequence of advanced social evolution. Curr. Opin. Insect Sci. 2016, 16, 64–68. [Google Scholar] [CrossRef]
- Yue, C.; Genersch, E. RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 2005, 86, 3419–3424. [Google Scholar] [CrossRef]
- Yañez, O.; Jaffé, R.; Jarosch, A.; Fries, I.; Robin, F.A.M.; Robert, J.P.; De Miranda, J.R. Deformed wing virus and drone mating flights in the honey bee (Apis mellifera): Implications for sexual transmission of a major honey bee virus. Apidologie 2012, 43, 17–30. [Google Scholar] [CrossRef]
- Duay, P.; De Jong, D.; Engels, W. Decreased flight performance and sperm production in drones of the honeybee (Apis mellifera) slightly infested by Varroa destructor mites during pupaldevelopment. Genet. Mol. Res. 2002, 1, 227–232. [Google Scholar] [PubMed]
- Peng, Y.; Baer-Imhoof, B.; Harvey Millar, A.; Baer, B. Consequences of Nosema apis infection for male honey bees and their fertility. Sci. Rep. 2015, 5, 10565. [Google Scholar] [CrossRef] [PubMed]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.L.; Villar, G.; Cheng, W.; Song, J.; Grozinger, C.M. Molecular, physiological and behavioral responses of honey bee (Apis mellifera) drones to infection with microsporidian parasites. J. Invertebr. Pathol. 2018, 155, 14–24. [Google Scholar] [CrossRef]
- Alaux, C.; Folschweiller, M.; McDonnell, C.; Beslay, D.; Cousin, M.; Dussaubat, C.; Brunet, J.L.; Conte, Y. Le Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). J. Invertebr. Pathol. 2011, 106, 380–385. [Google Scholar] [CrossRef]
- Yapici, N.; Kim, Y.-J.; Ribeiro, C.; Dickson, B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 2008, 451, 33–37. [Google Scholar] [CrossRef]
- Kohno, H.; Suenami, S.; Takeuchi, H.; Sasaki, T.; Kubo, T. Production of Knockout Mutants by CRISPR/Cas9 in the European Honeybee, Apis mellifera L. Zoolog. Sci. 2016, 33, 505–512. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Prostaglandins and their receptors in insect biology. Front. Endocrinol. 2011, 2, 1–11. [Google Scholar] [CrossRef]
- Worthington, A.M.; Jurenka, R.A.; Kelly, C.D. Mating for male-derived prostaglandin: A functional explanation for the increased fecundity of mated female crickets? J. Exp. Biol. 2015, 2720–2727. [Google Scholar] [CrossRef]
- Guarna, M.M.; Hoover, S.E.; Huxter, E.; Higo, H.; Moon, K.-M.; Domanski, D.; Bixby, M.E.F.; Melathopoulos, A.P.; Ibrahim, A.; Peirson, M.; et al. Peptide biomarkers used for the selective breeding of a complex polygenic trait in honey bees. Sci. Rep. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bixby, M.; Baylis, K.; Hoover, S.E.; Currie, R.W.; Andony, P.; Pernal, S.F.; Foster, L.J.; Guarna, M.M. Apiculture & Social Insects A Bio-Economic Case Study of Canadian Honey Bee (Hymenoptera: Apidae) Colonies: Marker-Assisted Selection (MAS) in Queen Breeding Affects Beekeeper Profits. J. Econ. Entomol. 2018, 110, 816–825. [Google Scholar] [CrossRef]

| Mating/Insemination Factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Queen Post-Mating Outcomes | CO2 vs. Virgins | CPM vs. Virgins | SDI and MDI vs. Virgins | SDI vs. MDI | Insemination Volume: 8 μL vs. 1 μL | Semen vs. Saline | Seminal Fluid vs. Hayes | Naturally Mated vs. Virgin |
| Reduced sexual receptivity? | Yes [99] | Yes [93] | unknown | unknown | Yes, ns [58] | Yes, ns [55] | Yes [93] | Yes [13,63,68] |
| Greater ovary activation? | Yes, ns [93]; Yes [96,99] | Yes [93] | unknown | unknown | Yes [58] | Yes [55] | unknown | Yes [72,73,96] |
| Enhanced worker retinue response? | Yes [93] | No [93] | Yes [47,48] | Yes [47,48] | Yes [50,51] | Yes [51] Yes, ns [49] | Yes [93] | Yes [47,49,67] |
| Modulated Mandibular gland pheromone production? | Yes [93] | Yes [93] | Yes [47] | Yes [47] | Yes [51] | Yes [51] | unknown | Yes [49,54,55,84] |
| Modulated Dufour’s gland pheromone production? | No [93] | No [93] | Yes [48] | Yes [48] | No Difference [51] | No Difference [51] | unknown | Yes [65] |
| # genes differentially expressed in brain out of all transcripts that were detected | 234/9091 [93] | 504/9091 [93] | unknown | unknown | unknown | 44/9850 [68] | unknown | 576/10,468 [57] 180/9850 [68] |
| # genes differentially expressed in ovaries out of all transcripts that were detected | unknown | unknown | unknown | unknown | unknown | unknown | unknown | 217/7377 [57] regulation of biogenic amine receptor genes [96] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brutscher, L.M.; Baer, B.; Niño, E.L. Putative Drone Copulation Factors Regulating Honey Bee (Apis mellifera) Queen Reproduction and Health: A Review. Insects 2019, 10, 8. https://doi.org/10.3390/insects10010008
Brutscher LM, Baer B, Niño EL. Putative Drone Copulation Factors Regulating Honey Bee (Apis mellifera) Queen Reproduction and Health: A Review. Insects. 2019; 10(1):8. https://doi.org/10.3390/insects10010008
Chicago/Turabian StyleBrutscher, Laura M., Boris Baer, and Elina L. Niño. 2019. "Putative Drone Copulation Factors Regulating Honey Bee (Apis mellifera) Queen Reproduction and Health: A Review" Insects 10, no. 1: 8. https://doi.org/10.3390/insects10010008
APA StyleBrutscher, L. M., Baer, B., & Niño, E. L. (2019). Putative Drone Copulation Factors Regulating Honey Bee (Apis mellifera) Queen Reproduction and Health: A Review. Insects, 10(1), 8. https://doi.org/10.3390/insects10010008