1. Introduction
The predatory mites
Galendromus occidentalis (Nesbitt) and
Amblydromella caudiglans (Schuster) are the most important and abundant predators of spider mites in Washington State apples [
1]. These predators have different life history characteristics, habitat preferences, and prey preferences, providing Washington State apple growers with slightly different biocontrol services [
1,
2,
3,
4,
5,
6]. Specifically,
A. caudiglans is a generalist predator capable of feeding on tetranychids, eriophyids, and pollen, with a preference for non-web-spinning tetranychids [
4,
7].
Galendromus occidentalis is a more specialized predator of web-spinning tetranychid spider mites (especially
Tetranychus spp.), although it will also consume other tetranychid species and eriophyids [
4]. Prior research indicates that
G. occidentalis and
A. caudiglans have similar pesticide sensitivity, although
A. caudiglans is more sensitive to older, broad-spectrum insecticides [
5,
8,
9]. Conservation of these native predatory mites is based around the judicious use of pesticides for the control of major pests, such as codling moth (
Cydia pomenella L.). Pesticides are chosen that are minimally harmful to
G. occidentalis. This has been a cornerstone of integrated mite management since the 1960s [
10]. The role of
A. caudiglans has become appreciated more recently and work is still needed to determine how to best conserve this phytoseiid [
1,
5].
Across cropping systems, the non-target impacts of herbicides on beneficial arthropods have been ignored [
11,
12,
13]. Although phytoseiids are well represented in pesticide selectivity studies [
14], recent reviews have found only a limited number of studies examining the effects of herbicides on this group [
12,
15]. These reviews indicated that herbicides have the potential to be just as toxic as insecticides, depending on the active ingredient tested [
12]. Predatory mites in orchards are known to benefit from ground cover because it provides shelter, floral resources, and alternative prey [
16,
17,
18,
19]. However, the negative impacts of bare ground may also be due to the harmful effects of herbicide residues. To date, only one study has examined how in-field herbicide applications impact orchard natural enemies. Applications of paraquat reduced abundance of the phytoseiid
Neoseiulus fallacis (Garman) and resulted in subsequent spider mite outbreaks [
20].
There have been no studies on how herbicides impact either
G. occidentalis or
A. caudiglans [
12,
15]. However, there are opportunities for these phytoseiids to encounter herbicides in the field.
Amblydromella caudiglans has been found to move between the canopy and the ground cover throughout the season and individuals collected from the canopy have been found with DNA from ground cover plants in their guts, likely from pollen consumption (Bergeron, unpublished). Additionally, higher populations of
A. caudiglans are associated with orchards with weedy herbicide strips (area directly under the trees) [
1]. Similar studies have not been performed for
G. occidentalis, but individuals have been found adjacent to orchards overwintering on common mullein (
Verbascum thapsus L.) [
21], indicating that it may also move up and down the canopy. It is also plausible that, as prey resources (i.e., spider mites such as
Tetranychus urticae Koch) move from the canopy to ground cover weeds, these specialized predators would follow.
Most herbicide labels recommend mixing with one or more adjuvants. In Washington, adjuvants are considered pesticides (Washington State Legislature: WAC 16-228-1400). How adjuvants affect pesticide toxicity for natural enemies is virtually unknown. However, work on
Pardosa spiders has demonstrated that adjuvants can decrease predatory activity when applied alone [
22] or in a mixture with glyphosate [
23]. Given the potential of adjuvants to increase the harm of herbicides and other pesticides to natural enemies, this line of research should be further investigated to improve conservation recommendations. This is particularly true for the phytoseiids; many adjuvants are oils, which are known to have at least minor non-target effects on predatory mites [
24,
25].
Given the uncertain future of glyphosate [
26,
27], research on non-target effects of herbicides is timely. Tree fruit growers in the northwestern U.S.A. are indicating that glufosinate will be their main glyphosate replacement product, but paraquat is also still available and has similar post-weed emergence burn-down. This is concerning for orchard conservation biological control because all current studies on glufosinate suggest it is much more harmful to natural enemies than glyphosate [
13,
15,
28,
29]. Paraquat can also cause substantial mortality in a variety of natural enemies [
30,
31,
32,
33]. Therefore, if glyphosate is phased out, there is potential for a shift to the use of herbicides, such as glufosinate and paraquat, that are disruptive to biological control of mites and other pests.
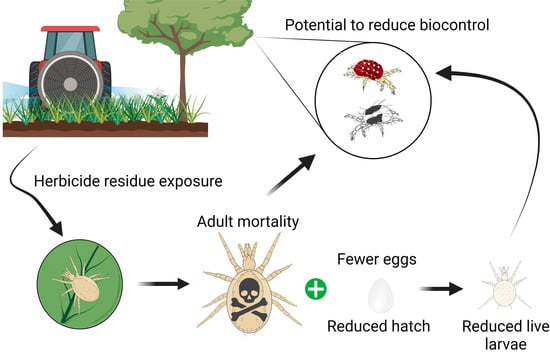
For Washington State apple growers, conservation of natural enemies may require judicious herbicide use. The purpose of this study was to determine the lethal and sublethal effects of freshly dried residues of common orchard herbicides and adjuvants independently, as well as the manufacturer’s recommended tank mixtures, on G. occidentalis and A. caudiglans. The results will be used to determine herbicide use recommendations for conserving these important mite predators.
4. Discussion
Herbicides clearly have the potential to disrupt biological control provided by predatory mites in apple orchards. Mortality caused by glufosinate and paraquat is comparable to that of broad-spectrum insecticides that are known to cause spider mite outbreaks in orchards [
5,
37]. Additionally, the sublethal effects of oxyfluorfen resulted in almost no viable offspring for treated females. However, there is the potential that “wild” strains of
G. occidentalis would respond to herbicides differently than the insectary population tested in this study, or that other populations of
A. caudiglans would respond differently. The insectary strain in particular may be more susceptible to pesticides in general than a field population. Therefore, this study only reflects first steps towards determining how herbicide applications will impact predatory mites in the field. Impacts of glufosinate, paraquat, and oxyfluorfen in particular should be further examined for their potential to increase pest mite damage in the field. These results are particularly concerning given that glufosinate and paraquat are the primary post-emergent alternatives to glyphosate. Because glyphosate resulted in little mortality and less substantial sublethal effects for both predators, increased use of other herbicides as a result of reduced glyphosate use is a probable concern for biological control.
The non-target effects of glufosinate on natural enemies in general are poorly understood, relative to other pesticides [
38]. The phytoseiids
Phytoseiulus persimilis Athias-Henriot,
N. fallacis, and
Amblyseius womersleyi Schicha experienced significant increases in mortality following treatment with glufosinate [
28,
29,
39]. In all studies on phytoseiids and glufosinate, the herbicide has been found harmful to the phytoseiid tested [
15]. In other natural enemies, results have been more varied. Little mortality was seen in
Chrysopa pallens Rambur or adult
Harmonia axyridis (Pallas) [
28]. However, glufosinate caused substantial mortality in
Orius strigicollis Poppius and some juvenile stages of
H. axyridis [
28]. In the parasitoid
Palmistichus elaeisis Delvare and LaSalle, glufosinate treatment reduced parasitism and emergence rates [
40]. In three species of spiders found in orchards, exposure to glufosinate residues did not cause any mortality [
13], but direct application increased mortality in
Pardosa agrestis (Westring) [
38]. The impact of glufosinate on a greater variety of natural enemies needs to be examined in the lab and the field, but the current trend indicates that glufosinate use could be particularly disruptive to integrated mite management.
Of the tested herbicides, paraquat is the only one to have been directly linked to outbreaks of secondary pests under field conditions [
20]. This has been attributed to its non-target effects on
N. fallacis [
30,
39,
41]. Laboratory studies on
Euseius hibicsci (Chant) also found that paraquat caused ~50% mortality and low fecundity when females were exposed to dry residues [
32]. While
N. fallacis is the primary predator of spider mites in northeastern U.S. orchards, this role is filled by
G. occidentalis and
A. caudiglans in the northwest [
42]. Therefore, there is a significant risk that paraquat use is equally harmful to northwestern mite biological control. Given this and the substantial human health risks of paraquat [
43], its use should be highly limited.
Although oxyfluorfen caused little direct mortality, significant sublethal effects on
A. caudiglans and
G. occidentalis were observed. Accumulation of sublethal effects via reduced fecundity and egg hatch reduced production of live larvae by treated females to nearly zero. Previous research on non-target effects of oxyfluorfen to
N. fallacis have reported high mortality (96%) [
39], while work on
P. persimilis suggests moderate toxicity (33–36% mortality) [
15]. Neither of these studies examined reproductive effects. Interestingly, various orchard-inhabiting spiders experienced relatively highly mortality after 48 h of exposure to residues, even though they had very little susceptibility to other herbicides tested, including paraquat and glufosinate [
13]. How oxyfluorfen affects natural enemy populations in orchards merits further study.
In general, 2,4-D has been found to be harmless to phytoseiids [
15], including
P. persimilis,
Amblyseius andersoni (Chant), and
Typhlodromus pyri Scheuten [
29,
44]. However, a study examining the effects of 2,4-D on
N. fallacis and
T. urticae found that it was over 4× as toxic to the predator than the pest, suggesting the potential for biological control disruption [
45]. Our study found that 2,4-D primarily impacts phytoseiids through reduced fecundity and egg hatch. Further studies on laboratory non-target effects and follow-up semi-field and field studies are needed to determine if 2,4-D alters predator:prey ratios.
Glyphosate is among the most studied herbicides due to growing consumer concerns about its overuse in agricultural and urban landscapes. For phytoseiids, impacts of glyphosate in laboratory studies have been highly variable [
15] based on both the species and formulation tested, with findings of both “highly toxic” [
32,
41] and “harmless” [
46]. Species that are typically considered more susceptible to pesticides (
Euseius spp.,
N. fallacis) were more sensitive to glyphosate, and those that are known to be less susceptible (
P. persimilis,
T. pyri) were those in the “harmless” category [
12,
15]. Given the extreme number of glyphosate formulations available [
47], it is not surprising that toxicity to phytoseiids would be equally variable. In honey bees, glyphosate as pure AI has been found to be non-toxic, but many of the surfactants and other additives in the formulation have known non-target effects [
47]. Because the present study and previous work have shown that glyphosate is less toxic to natural enemies than some of its alternatives, identifying formulations of glyphosate that are minimally harmful to beneficial insects and human health [
47] may be essential for successful IPM.
Few studies have examined non-target impacts of rimsulfuron and halosulfuron, which are both sulfonylurea herbicides. In one study, halosulfuron did not increase
P. persimilis mortality compared to the control when exposed by direct contact or residues [
15]. In spiders, neither herbicide caused any mortality even after five days of exposure to residues [
13]. However, both herbicides appeared to be an irritant to
Philodromus cespitum (Walckenaer), increasing its movement by over five-fold [
13]. Both herbicides also reduced prey consumption by
Pelegrina aeneola (Curtis) [
13]. There are no other studies in the literature on either halosulfuron’s or rimsulfuron’s non-target effects. However, research on other sulfonylureas indicates this group is among the least harmful herbicides to natural enemies [
48,
49,
50,
51].
Non-target effects of adjuvants on
G. occidentalis and
A. caudiglans were minimal. This study suggests that the non-ionic surfactant and methylated seed oil have the greatest impact on these two species, although significant effects were only observed in
G. occidentalis. These adjuvants are commonly referred to as activator compounds because they are designed for spreading, dispersion, emulsification, and increased penetration of the plant leaf surface to improve herbicide activity [
52,
53,
54,
55]. A possible explanation for the sublethal effects due to exposure with adjuvant may be found in the viscosity of methylated seed oil or the non-ionic surfactant; oils are known to be harmful to predatory mites. While adjuvants are not technically pesticides, some states within the U.S.A. (e.g., Washington) consider them pesticides for regulatory purposes. Unfortunately, there is very little research on the non-target effects of adjuvants on natural enemies, limiting our ability to confirm results from the present study. Given the research indicating that supposed inert ingredients can significantly harm beneficial insects [
23,
47], pesticide testing should increase its focus to also examine differences in formulation toxicity.
The mechanisms of toxicity for non-insecticides on non-target arthropods are virtually unstudied and the mode of action of herbicides on arthropods remains unknown [
56]. There are no consistent patterns regarding toxicity within a herbicide mode of action group [
14,
15]. Toxicity may be linked to the physical properties of the active ingredients, such as lipophilicity or volatility [
15]. In one study examining
T. urticae and
P. persimilis, inactive ingredients within the formulated product caused high viscosity, resulting in smothering of both species after contact exposure [
15]. This effect was not seen when the mites were exposed to residues [
15]. Glyphosate has been found to alter the endosymbiont community within a ladybeetle, and these changes were associated with reduced body weight [
57]. In a ground beetle, exposure to the herbicide pendimethalin resulted in decreased gut microbiome diversity and decreased abundance of various bacteria genera associated with metabolism and detoxification [
58]. Many additional toxicology studies will be necessary to determine how herbicide active ingredients and formulations cause non-target effects in arthropods.
In an orchard, the two predatory mites might differ in their potential for exposure to herbicides. In surveys of pear orchards,
A. caudiglans is more commonly found in the ground cover than
G. occidentalis (Schmidt-Jeffris, unpublished), increasing its exposure risk. Host plant preferences may also affect herbicide exposure.
Amblydromella caudiglans appears to prefer host plants with trichome-dense leaves [
1,
59], potentially because the trichomes serve as pollen traps and
A. caudiglans is a generalist phytoseiid that can sustain population growth on pollen diets [
7,
60,
61]. Trichomes can reduce pesticide penetration [
55], which could offer
A. caudiglans some protection on these leaf surfaces. There is evidence that
Galendromus occidentalis may also prefer trichome-dense host plants [
59], but other studies have suggested that this more specialist species may not have strong host plant preferences [
1,
62]. Multiple aspects of phytoseiid biology might alter how these predators respond to herbicides in the field.
Because our study used laboratory assays, it can only identify potentially harmful herbicides. Field studies are needed to confirm the effects of these herbicides on phytoseiid populations in orchards and to determine if the effects are significant enough to cause pest outbreaks. There are virtually no field studies examining how herbicide applications for weed management in crops impact natural enemy abundance and biological control services. This is likely because impacts of herbicide toxicity and loss of habitat, floral resources, and alternative prey provided by weeds are difficult to detangle. Because reducing weed management has been associated with increased phytoseiid abundance and biological control [
16,
17,
18,
19], the effects of various weed management practices should be tested to find combinations that reduce impact on natural enemies while still optimizing yield. Judicious weed control may be particularly important for conserving
A. caudiglans [
1]. Therefore, the creation of a resilient natural enemy community must incorporate alterations to how orchard ground cover is managed.