Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis and Preparation
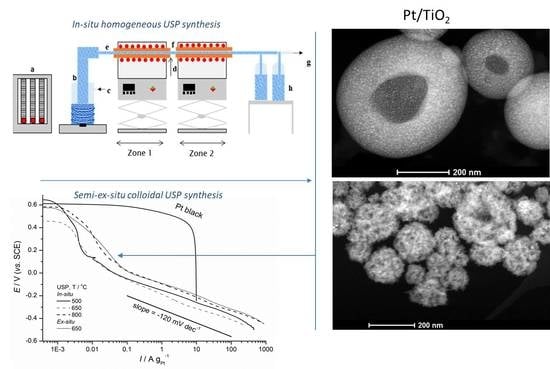
2.1.1. Synthesis of the Pt/TiO2 Powders
2.1.2. Preparation of Pt/TiO2 Coating on Glassy Carbon from Synthesized Powders
2.2. Material Characterization
2.2.1. Composition, Morphology, and Structural Characterization
2.2.2. Electrochemical Characterization
3. Results and Discussion
3.1. STEM and EDX Characterization of Pt/TiO2 Samples
3.1.1. USP Pt/TiO2 Samples Synthesized In-Situ
3.1.2. USP Pt/TiO2 Samples Synthesized Ex-Situ from Colloidal TiO2
3.2. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berber, M.R.; Hafez, I.H.; Fujigaya, T.; Nakashima, N. A highly durable fuel cell electrocatalyst based on double-polymer-coated carbon nanotubes. Sci. Rep. 2015, 5, 16711. [Google Scholar] [CrossRef]
- Wang, J. System integration, durability and reliability of fuel cells: Challenges and solutions. Appl. Energy 2017, 189, 460–479. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, S.-J.; Lee, J.-H.; Song, I.; Kim, N.-I.; Seo, Y.; Kim, K.B.; Lee, N.; Park, J.-Y. Degradation pattern prediction of a polymer electrolyte membrane fuel cell stack with series reliability structure via durability data of single cells. Appl. Energy 2014, 131, 48–55. [Google Scholar] [CrossRef]
- Wang, X.X.; Tan, Z.H.; Zeng, M.; Wang, J.N. Carbon nanocages: A new support material for Pt catalyst with remarkably high durability. Sci. Rep. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Gyergyek, S.; Li, Q.; Andersen, S.M. Evolution of the degradation mechanisms with the number of stress cycles during an accelerated stress test of carbon supported platinum nanoparticles. J. Electroanal. Chem. 2019, 838, 82–88. [Google Scholar] [CrossRef]
- Teran-Salgado, E.; Bahena-Uribe, D.; Márquez-Aguilar, P.A.; Reyes-Rodriguez, J.L.; Cruz-Silva, R.; Solorza-Feria, O. Platinum nanoparticles supported on electrochemically oxidized and exfoliated graphite for the oxygen reduction reaction. Electrochim. Acta 2019, 298, 172–185. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kwon, Y.; Kim, T.-W.; Kim, K.; Kim, H.J. Enhanced electrochemical oxygen reduction reaction performance with Pt nanocluster catalysts supported on microporous graphene-like 3D carbon. J. Electroanal. Chem. 2019, 838, 89–93. [Google Scholar] [CrossRef]
- Teng, X.; Yang, H. Synthesis and electrocatalytic property of cubic and spherical nanoparticles of cobalt platinum alloys. Front. Chem. Eng. China 2010, 4, 45–51. [Google Scholar] [CrossRef]
- Krstajić Pajić, M.N.; Stevanović, S.I.; Radmilović, V.V.; Gavrilović-Wohlmutherc, A.; Radmilović, V.R.; Gojković, S.L.; Jovanović, V.M. Shape evolution of carbon supported Pt nanopar-ticles: From synthesis to application. Appl. Catal. B Environ. 2016, 196, 174–184. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Chirea, M.; Altantzis, T.; Pastoriza-Santos, I.; Pe’rez-Juste, J.; Silva, F.; Bals, S.; Liz-Marzan, L.M. Dimethylformamide-mediated synthesis of water-soluble platinum nanodendrites for ethanol oxidation electrocatalysis†. Nanoscale 2013, 5, 4776–4784. [Google Scholar] [CrossRef]
- Gao, Q.; Gao, M.R.; Liu, J.W.; Chen, M.Y.; Cui, C.H.; Lia, H.H.; Yu, S.H. One-pot synthesis of branched palladium nanodendrites with superior electrocatalytic performance. Nanoscale 2013, 5, 3202–3207. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Wang, R.; Wang, H.; Luo, F.; Liao, S. Core–Shell-Structured Low-Platinum Electrocatalysts for Fuel Cell Applications. Electrochem. Energy Rev. 2018, 1, 324–387. [Google Scholar] [CrossRef]
- Jayawickrama, S.M.; Han, Z.; Kido, S.; Nakashima, N.; Fujigaya, T. Enhanced platinum utilization efficiency of polymer-coated carbon black as an electrocatalyst in polymer electrolyte membrane fuel cells. Electrochim. Acta 2019, 312, 349–357. [Google Scholar] [CrossRef]
- Devrim, Y.; Arıca, E.D. Multi-walled carbon nanotubes decorated by platinum catalyst for high temperature PEM fuel cell. Int. J. Hydrog. Energy 2019, 44, 18951–18966. [Google Scholar] [CrossRef]
- Berghian-Grosan, C.; Radu, T.; Biris, A.R.; Dan, M.; Voica, C.; Watanabe, F.; Biris, A.S.; Vulcu, A. Platinum nanoparticles coated by graphene layers: A low-metal loading catalyst for methanol oxidation in alkaline media. J. Energy Chem. 2020, 40, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Alcaide, F.; Álvarez, G.; Miguel, O.; Lázaro, M.J.; Moliner, R.; López-Cudero, A.; Solla-Gullón, J.; Herrero, E.; Aldaz, A. Pt supported on carbon nanofibers as electrocatalyst for low temperature polymer electrolyte membrane fuel cells. Electrochem. Commun. 2009, 11, 1081–1084. [Google Scholar] [CrossRef]
- Fraser, A.; Zhang, Z.; Merle, G.; Gbureck, U.; Ye, S.; Gostick, J.; Barralet, J. Composite Carbon Nanotube Microsphere Coatings for Use as Electrode Supports. Adv. Funct. Mater. 2018, 28, 1803713. [Google Scholar] [CrossRef]
- Lafforgue, C.; Zadick, A.; Dubau, L.; Maillard, F.; Chatenet, M. Selected Review of the Degradation of Pt and Pd-based Carbon-supported Electrocatalysts for Alkaline Fuel Cells: Towards Mechanisms of Degradation. Fuel Cells 2018, 18, 229–238. [Google Scholar] [CrossRef]
- Kreitmeier, S.; Wokaun, A.; Büchi, F.N. Local Catalyst Support Degradation during Polymer Electrolyte Fuel Cell Start-Up and Shutdown. J. Electrochem. Soc. 2012, 159, F787–F793. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wang, Y.; Ding, W.; Wu, R.; Li, L.; Qi, X.; Wei, Z. Study of the degradation mechanisms of carbon-supported platinum fuel cells catalyst via different accelerated stress test. J. Power Sources 2015, 273, 62–69. [Google Scholar] [CrossRef]
- Wu, Z.; Dang, D.; Tian, X. Designing Robust Support for Pt Alloy Nanoframes with Durable Oxygen Reduction Reaction Activity. ACS Appl. Mater. Interfaces 2019, 11, 9117–9124. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, Z.; Dang, D.; Wang, G.; Tian, X.; Yang, X. Three dimensional titanium molybdenum nitride nanowire assemblies as highly efficient and durable platinum support for methanol oxidation reaction. Electrochim. Acta 2019, 295, 50–57. [Google Scholar] [CrossRef]
- Huynh, T.T.; Pham, H.Q.; Nguyen, A.V.; Bach, L.G.; Ho, V.T.T. Advanced Nanoelectrocatalyst of Pt Nanoparticles Supported on Robust Ti0.7Ir0.3O2 as a Promising Catalyst for Fuel Cells. Ind. Eng. Chem. Res. 2019, 58, 675–684. [Google Scholar] [CrossRef]
- Liu, F.; Dang, D.; Tian, X. Platinum-decorated three dimensional titanium copper nitride architectures with durable methanol oxidation reaction activity. J. Hydrog. Energy 2019, 44, 8415–8424. [Google Scholar] [CrossRef]
- Kim, S.-W.; Han, T.H.; Kim, J.; Gwon, H.; Moon, H.-S.; Kang, S.-W.; Kim, S.O.; Kang, K. Fabrication and Electrochemical Characterization of TiO2 Three-Dimensional Nanonetwork Based on Peptide Assembly. ACS Nano 2009, 3, 1085–1090. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Fovet, Y.; Gal, J.Y.; Toumelin-Chemla, F. Influence of pH and fluoride concentration on titanium passivating layer: Stability of titanium dioxide. Talanta 2001, 53, 1053–1063. [Google Scholar] [CrossRef]
- Lewera, A.; Timperman, L.; Roguska, A.; Alonso-Vante, N. Metal-support interactions between nanosized Pt and metal oxides (WO3 and TiO2) studied using X-ray photoelectron spectroscopy. J. Phys. Chem. C 2011, 115, 20153–20159. [Google Scholar] [CrossRef]
- Dulub, O.; Hebenstreit, W.; Diebold, U. Imaging cluster surfaces with atomic resolution: The strong metal-support interaction state of Pt supported on TiO2 (110). Phys. Rev. Lett. 2000, 84, 3646–3649. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-S.; Zeid, A.; Kim, Y.-T. Additive treatment effect of TiO2 as supports for Pt-based electrocatalysts on oxygen reduction reaction activity. Electrochim. Acta 2010, 55, 3628–3633. [Google Scholar] [CrossRef]
- Wang, Y.; Mohamedi, M. Hierarchically organized nanostructured TiO2/Pt on microfibrous carbon paper substrate for ethanol fuel cell reaction. Int. J. Hydrog. Energy 2017, 42, 22796–22804. [Google Scholar] [CrossRef]
- Amer, M.S.; Ghanem, M.A.; Al-Mayouf, A.M.; Prabhakarn, A.N.H. Low-loading of oxidized platinum nanoparticles into mesoporous titanium dioxide for effective and durable hydrogen evolution in acidic media. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Al-Mayouf, A.M.; Ghanem, M.A.; AlHoshan, M.S.; Singh, J.P.; Al-Suhybani, A.A. Chemical Deposition and Electrocatalytic Activity of Platinum Nanoparticles Supported on TiO2 Nanotubes. Int. J. Electrochem. Sci. 2013, 8, 2468–2478. [Google Scholar]
- Guo, P.; Xu, W.; Zhu, S.; Yang, X.; Inoue, A. Preparation and electrocatalytic performance of the Pt supported on the alkali-treated nanoporous TiO2 material. Ionics 2015, 21, 2863–2869. [Google Scholar] [CrossRef]
- Košević, M.; Šekularac, G.; Živković, L.; Panić, V.; Nikolić, B. TiO2 From Colloidal Dispersion as Support in Pt/TiO2 Nanocomposite for Electrochemical Applications. Croat. Chem. Acta 2017, 90, 251–258. [Google Scholar] [CrossRef]
- Fugare, B.Y.; Lokhande, B.J. Study on structural, morphological, electrochemical and corrosion properties of mesoporous RuO2 thin films prepared by ultrasonic spray pyrolysis for supercapacitor electrode application. Mater. Sci. Semicond. Process. 2017, 71, 121–127. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Alkan, G.; Milošević, O.; Rabanal, M.E.; Friedrich, B. Synthesis and characterisation of spherical core-shell Ag/ZnO nanocomposites using single and two—Steps ultrasonic spray pyrolysis (USP). Catal. Today 2019, 321–322, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Alkan, G.; Diaz, F.; Matula, G.; Stopic, S.; Friedrich, B. Scaling up of nanopowder collection in the process of ultrasonic spray pyrolysis. World Metall.-ERZMETALL 2017, 70, 97–101. [Google Scholar]
- Mata, V.; Maldonado, A.; Olvera, M.L. Deposition of ZnO thin films by ultrasonic spray pyrolysis technique. Effect of the milling speed and time and its application in photocatalysis. Mater. Sci. Semicond. Process. 2018, 75, 288–295. [Google Scholar] [CrossRef]
- Liang, F.; Chen, S.; Xie, W.; Zou, C. The decoration of Nb-doped TiO2 microspheres by reduced graphene oxide for enhanced CO gas sensing. J. Phys. Chem. Solids 2018, 114, 195–200. [Google Scholar] [CrossRef]
- Alkan, G.; Rudolf, R.; Bogovic, J.; Jenko, D.; Friedrich, B. Structure and Formation Model of Ag/TiO2 and Au/TiO2 Nanoparticles Synthesized through Ultrasonic Spray Pyrolysis. Metals 2017, 7, 389. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Mo, Y.; Vukmirovic, M.B.; Klie, R.; Sasaki, K.; Adzic, R.R. Platinum Monolayer Electrocatalysts for O2 Reduction: Pt Monolayer on Pd(111) and on Carbon-Supported Pd Nanoparticles. J. Phys. Chem. B 2004, 108, 10955–10964. [Google Scholar] [CrossRef]
- Esmaeilifar, A.; Rowshanzamir, S.; Eikani, M.H.; Ghazanfari, E. Synthesis methods of low-Pt-loading electrocatalysts for proton exchange membrane fuel cell systems. Energy 2010, 35, 3941–3957. [Google Scholar] [CrossRef]
- Arici, E.; Kaplan, B.Y.; Mert, A.M.; Gursel, S.A.; Kinayyigit, S. An effective electrocatalyst based on platinum nanoparticles supported with graphene nanoplatelets and carbon black hybrid for PEM fuel cell. Int. J. Hydrog. Energy 2019, 44, 14175–14183. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, M.; Xia, M.; Wang, M.; Wang, X. An effective and durable interface structure design for oxygen reduction and methanol oxidation electrocatalyst. Appl. Surf. Sci. 2019, 487, 655–663. [Google Scholar] [CrossRef]
- Huang, W.; Wang, H.; Zhou, J.; Wang, J.; Duchesne, P.N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F.; Zeng, M.; et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum–nickel hydroxide–graphene. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Jakšić, M.M. Advances in electrocatalysis for hydrogen evolution in the light of the Brewer-Engel valence-bond theory. J. Mol. Catal. 1986, 38, 161–202. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, C.; Zhang, X.; Li, Y.; Ma, H.; Liu, Y. Control over energy level match in Keggin polyoxometallate-TiO2 microspheres for multielectron photocatalytic reactions. Appl. Catal. B 2018, 234, 79–89. [Google Scholar] [CrossRef]
- Yang, J.; Wang, G.; Wang, D.; Liu, C.; Zhang, Z. A self-cleaning coating material of TiO2 porous microspheres/cement composite with high-efficient photocatalytic depollution performance. Mater. Lett. 2017, 200, 1–5. [Google Scholar] [CrossRef]
- Stopic, S.; Friedrich, B.; Schroeder, M.; Weirich, T.E. Synthesis of TiO2 core/RuO2 shell particles using multistep ultrasonic spray pyrolysis. Mater. Res. Bull. 2013, 48, 3633–3635. [Google Scholar] [CrossRef]
- Kundu, M.K.; Bhowmik, T.; Mishra, R.; Barman, S. Pt Nanostructures/N-Doped Carbon hybrid, an Efficient Catalyst for Hydrogen Evolution/Oxidation Reactions: Enhancing its Base Media Activity through Bi-functionality of the Catalyst. ChemSusChem 2018, 11, 2388–2401. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, P.; Vinod Selvaganesh, S.; Sarathi, L.; Santoshkumar, D.B. Rutile TiO2 Supported Pt as Stable Electrocatalyst for Improved Oxygen Reduction Reaction and Durability in Polymer Electrolyte Fuel Cell. Electrocatalysis 2016, 7, 495–506. [Google Scholar] [CrossRef]
- Chiwata, M.; Kakinuma, K.; Wakisaka, M.; Uchida, M.; Deki, S.; Watanabe, M.; Uchida, H. Oxygen Reduction Reaction Activity and Durability of Pt Catalysts Supported on Titanium Carbide. Catalysts 2015, 5, 966–980. [Google Scholar] [CrossRef] [Green Version]
- Antoine, O.; Bultel, Y.; Durand, R. Oxygen reduction reaction kinetics and mechanism on platinum nanoparticles inside Nafion®. J. Electroanal. Chem 2001, 499, 85–94. [Google Scholar] [CrossRef]
- Creţu, R.; Kellenberger, A.; Vaszilcsin, N. Enhancement of hydrogen evolution reaction on platinum cathode by proton carriers. Int. J. Hydrog. Energy 2013, 38, 11685–11694. [Google Scholar] [CrossRef]








| Zone | Nominal Pt Loading, Mass % | Found Pt Loading Mass % | RelativeDecrease, % |
|---|---|---|---|
| Bright (outer side of the sphere wall, Figure 2a, area 2) | 20 | 17.1 | 14.5 |
| Dark (inner side of the sphere wall, Figure 2a, area 1) | 15.4 | 9.94 |
| EDX Sampling | Nominal Pt Loading, 20 mass% | Sphere Diameter, nm |
|---|---|---|
| Figure 2b, area 1 | 22.3 | 194 |
| Figure 2b, point 1/point 2 | 3.2/25.8 | |
| Figure 2c, area right | 15.2 | 198 |
| EDX Sampling | Mass % | Nominal Pt Loading, mass% | Sphere Diameter, nm |
|---|---|---|---|
| Figure 2c, area right | 20 | 15.2 | 198 |
| Figure 2c, area left | 5 | 3.0 | 278 |
| Sampling Site | USP Temperature, °C | Pt Loading of 20 mass % |
|---|---|---|
| Figure 4a, point 1 | 500 | 48.0 |
| Figure 4a, line 1 | 500 | 27.6 |
| Figure 4b, point 1 | 800 | 43.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Košević, M.G.; Zarić, M.M.; Stopić, S.R.; Stevanović, J.S.; Weirich, T.E.; Friedrich, B.G.; Panić, V.V. Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis. Metals 2020, 10, 11. https://doi.org/10.3390/met10010011
Košević MG, Zarić MM, Stopić SR, Stevanović JS, Weirich TE, Friedrich BG, Panić VV. Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis. Metals. 2020; 10(1):11. https://doi.org/10.3390/met10010011
Chicago/Turabian StyleKošević, Milica G., Milana M. Zarić, Srećko R. Stopić, Jasmina S. Stevanović, Thomas E. Weirich, Bernd G. Friedrich, and Vladimir V. Panić. 2020. "Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis" Metals 10, no. 1: 11. https://doi.org/10.3390/met10010011
APA StyleKošević, M. G., Zarić, M. M., Stopić, S. R., Stevanović, J. S., Weirich, T. E., Friedrich, B. G., & Panić, V. V. (2020). Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis. Metals, 10(1), 11. https://doi.org/10.3390/met10010011