1. Introduction
The understanding of the dissolution and re-precipitation of Nb, Ti and or Ti-Nb carbo-nitrides during the high-temperature processing of austenite is of essential importance for the microstructural refinement of austenite. During the hot deformation of High-Strength Low-Alloy (HSLA) steel, there are a series of well-known steps; (1) reheating, (2) roughing deformation, and (3) finishing deformation. This paper focuses on the impact of the precipitate composition and morphology on two crucial aspects: precipitate dissolution and control of the grain-coarsening behavior of austenite. All are applied to the reheating of a Ti-Nb microalloyed linepipe steel.
The purpose of this work was to study the feasibility of producing similar as-reheated austenitic grain size using two steels with different Nb levels at constant Ti content. The impact of simple precipitation morphology on the grain-coarsening behavior of austenite during reheating follows the kinetics of precipitation dissolution. However, when the morphology of the precipitates is more complex, the dissolution of the precipitates does not follow the standard equilibrium predictions.
It is well-known that the type of precipitation species and precipitation stability are key for Prior-Austenite Grain Size (PAGS) control. Precipitates exert pinning forces on GBs, limiting their mobility [
1,
2,
3,
4,
5,
6]. Hence, when precipitates dissolve or coarsen, GBs detach from them and grain coarsening occurs. This gives importance to the dissolution of particles and the factors that affect it.
Previous research by Gong et al. have shown the higher stability of Nb-Ti carbo-nitrides as compared to Nb-only carbo-nitrides [
7]. Gong’s work points out the faster dissolution rate of NbC as compared to TiNb-CN as the reason for this behavior, without considering the structural make-up of the particles as a factor. Gong et al. also point out that PAGS must be calculated considering solute drag effects to get accurate predictions. In a work by Ringer et al, precipitate shape has been proposed as a factor of the GB-pinning force [
8]. It is suggested that, in the process of unpinning, the precipitate must first rotate to acquire coherency with the new grain, delaying grain growth in the process. Shape is a clear difference when comparing NbTi carbonitride cuboidal precipitates to Nb-only carbonitrides of more rounded shape. NbC has previously been reported to nucleate at pre-existing TiN [
9,
10,
11]. If this heterogeneous formation of NbC on TiN particles happens during casting, as suggested by several authors [
11,
12], the dissolution process during reheating could be retarded. Slower dissolution kinetics, for instance, can be utilized for better PAGS control with lower levels of microalloying elements. Research has already shown epitaxial NbC on TiN as an effective precipitate for grain size control [
13,
14].
The complexity of TiN-NbC particles’ morphology and composition may slow down dissolution kinetics. The stability of NbC interfaces to TiN and TiC has been studied using ab initio calculations; researchers have shown how the NbC/TiC interface is the most stable [
15]. They report a smaller mismatch of the NbC/TiC interface when compared to the NbC/TiN interface, as well as a lower interface energy 0.75 J·m
−2, 3.6 times smaller than the NbC/TiN interface. This means that as the core-particle loses N and gains C, coherency improves between the layers. Hence, the interface between these two compounds stabilizes as they interdiffuse.
Not-complex particles may dissolve first. Then, microalloy elements in solution can interact with GB by forming solute-rich atmospheres around them. These atmospheres lower GB energy, reducing GB mobility and producing the well-known solute-drag effect.
The major goal of this work was to compare and understand the impact of the precipitate morphology and dissolution behavior on grain coarsening in an HSLA-LP steel.
2. Materials and Methods
The two alloys in
Table 1 were studied. The Nb content was the only contrast in chemical composition. The alloys were produced as lab-heats at a partner industrial facility, hot-rolled down to 19 mm thickness and air-cooled. The chemical composition was provided by our partner steelmaking laboratory through Inductively Coupled Plasma Mass Spectroscopy. The initial hot rolled microstructure for both steels was Ferrite (F) and Pearlite (P).
By assuming equilibrium conditions, the empirical solubility products of the possible precipitates can be used to find their correspondent volume fraction, f
v. Volume fraction was obtained as a function of temperature, based on the chemical compositions. A model that considers the mutual solubility of carbonitride was compared to a simple-precipitates model. See
Appendix A and
Appendix B for details.
Austenitization was done in vacuum quartz capsules containing one piece of each alloy. The capsules were austenitized to 1150, 1200, 1250 and 1300 °C for 1 h, then water quenched. The resulting specimens were cut in half, so half was used for Prior-Austenitic Grain Size measurements, and the other half for precipitate and dissolution assessment. The PAGS was revealed by first tempering the specimens at 600 °C for 24 h, followed by picral solution etching. The picral solution contained 100 mL of distilled de-ionized water, picric acid to saturation, 2 g of sodium dodecylbenzenesulfonate and six drops of hydrochloric acid. Etching was done at 90 °C.
PAGS measurements were done utilizing the free software ImageJ (1.52a, National Institutes of Health, Bethesda, MD, USA), by manually circling grains to obtain the average Feret diameter. Precipitates were quantified based on TEM analysis obtained from random locations of the specimens. Two sets of TEM micrographs were used with magnifications 2000× and 20,000×, to obtain representative samples of the smallest and the biggest particles. The software ImageJ was used to measure particle size and area fraction. The area fraction was converted to volume fraction by assuming the micrographs are representative of a volume as thick as an average precipitate.
Another method for revealing PAGS was reconstruction of austenitic EBSD maps, based on the quenched martensitic specimens. The specimens were ground, polished, vibro-polished for 3 h, then cleaned in an ultrasonic bath of ethanol for 10 min before scanning. EBSD was done using a 20 kV, 13 nA beam, in a hexagonal pattern with 0.3 μm step size. The martensitic EBSD maps were processed to reconstruct prior-austenite maps. MATLAB (R2017b, The Mathworks Inc., Natick, MA, USA) with MTEX toolbox was used, following the procedure proposed by Nyyssönnen, et al. [
16,
17]. This procedure starts by determining the experimental Orientation Relationship (OR) between martensite and its parent austenite. Kurdjumov-Sachs OR is first assumed, then followed by an iterative comparison of adjacent grains; this determines the experimental OR. This OR is then used for re-constructing the prior-austenite orientation map.
Thin foil specimens for TEM were produced by grinding specimens down to 100 μm thickness with 1200 grit sandpaper. Further grinding to 10–20 μm was done in a Fischione Model 200 dimpling grinder (Fischione Instruments, Export, PA, USA) using 3 nm diamond abrasive paste. Electropolishing was done on the thin foils using a Struers TenuPol-5 (Struers Inc., Cleveland, OH, USA) with 5% perchloric acid solution as electrolyte at −25 to −15 °C. Carbon replica extractions were obtained by evaporating 10–15 nm C-film on 3% Nital etched surfaces of the specimens. The film was then removed by immersion in 10% Nital and supported on copper grids for analysis.
Light microscopy was done in a Keyence Microscope (Keyence Corporation of America, Itasca, IL, USA). SEM analysis was done in a Zeiss Sigma 50vp FEG-SEM (Zeiss International, Oberkochen, Germany) equipped with Oxford Energy Dispersive Spectrometer (EDS) detector (Oxford Instruments, Abingdon, UK). EDS was used as qualitative method, utilizing only the factory-preset standardization of the Aztec software (V3.2 SP1, Oxford Instruments, Abingdon, UK). EBSD was done in a FEI Scios FIB/SEM Dual Beam system (Thermo Fisher, Hillsboro, OR, USA) with EDAX EBSD camera and TEAM software (V4.3, EDAX Inc., Mahwah, NJ, USA). TEM was done in a JEOL JEM2100F (JEOL Ltd., Tokyo, Japan) using 200 kV beam.
Author Contributions
Conceptualization, G.S.-B., M.M. and C.I.G.; methodology, G.S.-B.; validation, G.S.-B., M.M. and C.I.G.; formal analysis, G.S.-B.; investigation, G.S.-B. and M.M.; resources, M.M. and C.I.G.; data curation, G.S.-B.; writing—original draft preparation, G.S.-B.; writing—review and editing, C.I.G.; visualization, G.S.-B.; supervision, C.I.G.; project administration, C.I.G.; funding acquisition, C.I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Pennsylvania Department of Community and Economic Development CMU Subcontract 1060145-406196—Pitt I# 0062204; Programa para el Desarrollo Profesional Docente, PRODEP DSA/103.5/15/9555; United States Steel Corporation, and the APC was funded by The Ferrous Physical Metallurgy research group at University of Pittsburgh.
Acknowledgments
We are grateful for the support of: PRODEP México, Universidad Veracruzana, Roberto Rocca Education Program and Pennsylvania Department of Community and Economic Development. The authors want to thank the Ferrous Physical Metallurgy Research Group fellows and collaborators at University of Pittsburgh, as much as to collaborators at United States Steel Corporation: Justin Bryan, Debra Giansante, Andrew Warble and Christopher Snyder.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
The calculations for volume fractions were done in the following manner. Let us first consider the solubility products of the precipitates involved. The solubility product denotes the equilibrium concentrations of the alloying elements at a given temperature. For instance, the concentrations of M and N in equilibrium with the precipitate M
mN
n are given by
and
are mole fractions of M and N in solution and K is the solubility product [
27]. The solubility product changes with temperature, allowing for dissolution at higher temperature.
where ∆H represents the precipitate’s enthalpy of formation in the matrix and K
0 is a constant. Empirical adaptations use weight percentages instead of molar fractions of the solutes, and express the solubility product, Ks, as
where T, is the temperature of the alloy and the constants A, and B, are experimentally determined. These values were collected for the involved precipitates in an austenitic matrix and, combined with mass balance equations, a series of quadratic equations was produced. Then, these equations were solved in order of precipitate stability as expected from Gibbs’ free energy of formation.
As the alloy cools down, for instance, the preferable nitride is TiN. After TiN forms, the remaining N is available to form AlN, NbN and VN, in that preferential order. Similarly, for carbides, the preferential order for carbon would be TiC, NbC, VC, MoC. Following that order, the system of equations was solved for the mass fraction of every element that is in precipitate form at temperature intervals of 10 °C, from 200 to 1500 °C.
Cooling from high temperatures, the first precipitate to be formed would be TiN. Hence, after all the possible TiN has been formed and equilibrium is reached, the following equations must hold true
where X
T is the total mass fraction content in the bulk composition of species X,
is the mass fraction of X that remains in solid solution and X
TiN is the mass fraction of the species X in the form of TiN. Now, considering the stoichiometric mass ratio of TiN and following the same nomenclature, Equation (A4) can be re-written as
The solid solution contents of N and Ti in equilibrium with TiN are related through the solubility product, K
TiN. So, substituting Ti
TiN from Equation (A1) into Equation (A6), we can write
Now, notice that N
T and Ti
T are known fractions from our bulk composition, and K
TiN is known as a function of temperature from experimental reports in the literature (See
Table A1).
Table A1.
Solubility products of precipitates of interest in Austenite.
| | Reference |
|---|
| A | B |
|---|
| 3.42 | 7900 | Gladman [28] |
| 2.8 | 8500 | Gladman [28] |
| 5.33 | 10,475 | Gladman [28] |
| 3.82 | 15,020 | Gladman [28] |
| 1.8 | 7750 | Gao-Baker [2] |
| 4.45 | 6560 | Turkdogan [29,30] |
| 2.86 | 7700 | Turkdogan [29,30] |
| 1.29 | 523 | Pavlina, et al. [31] |
Hence, Equation (A7) can be rearranged and solved for
as a quadratic equation for every temperature of interest.
Once is found as the solid solution concentration of nitrogen in equilibrium with TiN, the simple mass balance from Equation (A6) helps us find NTiN, the amount of N in TiN. This way, the amount of TiN present at every temperature during cooling is found using the stoichiometric mass ratio.
Calculations for the mass fraction of every precipitate followed this method. Every new calculation considered new mass balance equations, accounting for the mass already precipitated in the higher temperature stable precipitates. For instance, when calculating MoC mass fraction, the mass balance equation for carbon must consider that precipitations of TiC, NbC and VC have already happened. Hence it must be written
When MoC precipitates, however, all other considered carbides are already present, so the amount of carbon present in each is known. Hence, the only unknowns in this equation are CMoC and .
Appendix B
Solubility product models that consider the complexity of precipitates mutual solubility have been proposed elsewhere [
32,
33,
34]. The present work used a simplified system of equations, inspired by Xu, et al.’s work. A system of 21 equations was reduced to eight equations and eight unknowns. The eight main equations came from two equations of activity of the precipitates, and six equations of the mass balance of C, N, Ti, Nb, V and Al. These eight equations were fed with the other 13 equations, relating the solubility products of seven precipitates (AlN, TiC, TiN, NbC, NbN, VC and VN) and the activities of the six involved components.
The first two main equations state assumptions of activities equal to one, for a stable precipitate in the matrix. The mutual solubility of TiC, TiN, NbC, NbN, VC and VN is considered, so the combined activity of this complex precipitate is 1, whereas AlN is insoluble, so its activity remains 1:
Mass balance provided the other six main equations. For every element, the amount present in every phase must add up to the total bulk content of that component, M
T. Hence, the molar fractions, X, of three phases were considered: the matrix, the volume fraction of aluminum nitride, X
AlN, and the molar fraction of the mutually soluble precipitates, X
cmplx.
At this point, there are 14 unknowns (six activities, two molar fractions and six concentrations) that outnumber the eight equations, but the following equations can be considered to simplify the system. The activities of all considered nitrides and carbides can be replaced by functions of temperature and the activities of the individual elements, utilizing the solubility products, K.
Therefore, the activity of every element may be replaced by the product of the activity coefficient,
, of the element, and that element’s concentration in solution
.
can be written as a function of concentrations and Wagner interaction coefficients,
, of every component on a given element. These coefficients can be found in the literature
Substituting activities from Equations (A25)–(A30) into Equations (A18)–(A24), and then substitute (A18)–(A24) into (A10)–(A17) transforms Equations (A10)–(A17) into a system of eight equations with eight unknowns. Furthermore, if the solid solution is regarded as dilute, due to the very small amounts of solutes, the activities of the individual components can be approximated to their concentration in solid solution. This transforms Equations (A10)–(A17) into a much simpler system of eight equations and eight unknowns (six concentrations and two molar fractions):
This system has numerous sets of solutions for a given temperature. Many of these solutions include complex and negative numbers, others fail to provide concentration values between zero and the bulk content of a component. Discretion was used to choose the right solution and obtain a reliable trend. The system of equations was solved with Wolfram Mathematica computing software.
References
- Cuddy, L.J.; Raley, J.C. Austenite grain coarsening in microalloyed steels. Metall. Trans. A 1983, 14, 1989–1995. [Google Scholar] [CrossRef]
- Gao, N.; Baker, T.N. Austenite Grain Growth Behaviour of Microalloyed Al–V–N and Al–V–Ti–N Steels. ISIJ Int. 1998, 38, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Gladman, T. On the theory of the effect of precipitate particles on grain growth in metals. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1966, 294, 298–309. [Google Scholar]
- Rios, P.R. On the relationship between pinning force and limiting grain radius. Scr. Mater. 1996, 34, 1185–1188. [Google Scholar] [CrossRef]
- Rios, P.R. A theory for grain boundary pinning by particles. Acta Metall. 1987, 35, 2805–2814. [Google Scholar] [CrossRef]
- Nishizawa, T.; Ohnuma, I.; Ishida, K. Examination of the Zener relationship between grain size and particle dispersion. Mater. Trans. JIM 1997, 38, 950–956. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Ringer, S.P.; Li, W.B.; Easterling, K.E. On the interaction and pinning of grain boundaries by cubic shaped precipitate particles. Acta Metall. 1989, 37, 831–841. [Google Scholar] [CrossRef]
- Hong, S.G.; Jun, H.J.; Kang, K.B.; Park, C.G. Evolution of precipitates in the Nb-Ti-V microalloyed HSLA steels during reheating. Scr. Mater. 2003, 48, 1201–1206. [Google Scholar] [CrossRef]
- Ma, X.; Miao, C.; Langelier, B.; Subramanian, S. Suppression of strain-induced precipitation of NbC by epitaxial growth of NbC on pre-existing TiN in Nb-Ti microalloyed steel. Mater. Des. 2017, 132, 244–249. [Google Scholar] [CrossRef]
- Craven, A.J.; He, K.; Garvie, L.A.J.; Baker, T.N. Complex heterogeneous precipitation in titanium–niobium microalloyed Al-killed HSLA steels—I. (Ti,Nb)(C,N) particles. Acta Mater. 2000, 48, 3857–3868. [Google Scholar] [CrossRef]
- Wang, R.; Garcia, C.I.; Hua, M.; Cho, K.; Zhang, H.; DeArdo, A.J. Microstructure and Precipitation Behavior of Nb, Ti Complex Microalloyed Steel Produced by Compact Strip Processing. ISIJ Int. 2006, 46, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.V.; Xiaoping, M.; Rehman, K. Austenite Grain Size Control in Upstream Processing of Niobium Microalloyed Steels by Nano-Scale Precipitate Engineering of TiN-NbC Composite. In Energy Materials 2014; Springer: Cham, Switzerland, 2014; pp. 639–650. [Google Scholar]
- Graux, A.; Cazottes, S.; De Castro, D.; San Martín, D.; Capdevila, C.; Cabrera, J.M.; Molas, S.; Schreiber, S.; Mirković, D.; Danoix, F.; et al. Precipitation and grain growth modelling in Ti-Nb microalloyed steels. Materialia 2019, 5, 100233. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Huihui, X. First-principles study of NbC heterogeneous nucleation on TiC vs. TiN in microalloy steel. Ironmak. Steelmak. 2018. [Google Scholar] [CrossRef]
- Nyyssönen, T.; Isakov, M.; Peura, P.; Kuokkala, V.-T. Iterative Determination of the Orientation Relationship Between Austenite and Martensite from a Large Amount of Grain Pair Misorientations. Metall. Mater. Trans. A 2016, 47, 2587–2590. [Google Scholar] [CrossRef]
- Nyyssönen, T.; Peura, P.; Kuokkala, V.-T. Crystallography, Morphology, and Martensite Transformation of Prior Austenite in Intercritically Annealed High-Aluminum Steel. Metall. Mater. Trans. A 2018, 49, 6426–6441. [Google Scholar] [CrossRef]
- Palmiere, E.J.; Garcia, C.I.; DeArdo, A.J. The influence of niobium supersaturation in austenite on the static recrystallization behavior of low carbon microalloyed steels. Metall. Mater. Trans. A 1996, 27, 951–960. [Google Scholar] [CrossRef]
- Blancas, V. A New View of the Grain-Coarsening Behavior of Austenite in Ti-Microalloyed Low-Carbon Steels; University of Pittsburgh: Pittsburgh, PA, USA, 2016. [Google Scholar]
- Hayakawa, Y.; Szpunar, J.A. The role of grain boundary character distribution in secondary recrystallization of electrical steels. Acta Mater. 1997, 45, 1285–1295. [Google Scholar] [CrossRef]
- Alogab, K.A.; Matlock, D.K.; Speer, J.G.; Kleebe, H.J. The Influence of Niobium Microalloying on Austenite Grain Coarsening Behavior of Ti-modified SAE 8620 Steel. ISIJ Int. 2007, 47, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Charleux, M.; Poole, W.J.; Militzer, M.; Deschamps, A. Precipitation Behavior and its Effect on Strengthening of an HSLA-NbTi Steel. Metall. Mater. Trans. A 2001, 32, 1635–1648. [Google Scholar] [CrossRef]
- Karmakar, A.; Kundu, S.; Roy, S.; Neogy, S.; Srivastava, D.; Chakrabarti, D. Effect of microalloying elements on austenite grain growth in Nb–Ti and Nb–V steels. Mater. Sci. Technol. 2014, 30, 653–664. [Google Scholar] [CrossRef]
- AlOgab, K.; Matlock, D.; Speer, J.; Kleebe, H. The Effects of Heating Rate on Austenite Grain Growth in a Ti-modified SAE 8620 Steel with Controlled Niobium Additions. ISIJ Int. 2007, 47, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Kirkaldy, J.S. Carbonitride precipitate growth in titanium/niobium microalloyed steels. Metall. Trans. A 1991, 22, 1511–1524. [Google Scholar] [CrossRef]
- Hutchinson, C.R.; Zurob, H.S.; Sinclair, C.W.; Brechet, Y.J.M. The comparative effectiveness of Nb solute and NbC precipitates at impeding grain-boundary motion in Nb steels. Scr. Mater. 2008, 59, 635–637. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M. Phase Transformations in Metals and Alloys, (Revised Reprint); CRC press: New York, NY, USA, 2009. [Google Scholar]
- Gladman, T. Precipitation hardening in metals. Mater. Sci. Technol. 1999, 15, 30–36. [Google Scholar] [CrossRef]
- Turkdogan, E. Causes and effects of nitride and carbonitride precipitation during continuous casting. Iron Steelmak. 1989, 16, 61. [Google Scholar]
- Homsher, C.N. Determination of the Non-Recrystallization Temperature (Tnr) in Multiple Microalloyed Steels; Colorado School of Mines: Denver, CL, USA, 2013. [Google Scholar]
- Pavlina, E.J.; Speer, J.G.; Van Tyne, C.J. Equilibrium solubility products of molybdenum carbide and tungsten carbide in iron. Scr. Mater. 2012, 66, 243–246. [Google Scholar] [CrossRef]
- Xu, K.; Thomas, B.G.; O’malley, R. Equilibrium Model of Precipitation in Microalloyed Steels. Metall. Mater. Trans. A 2011, 42, 524–539. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.R. A simple method to determine the complex carbonitride composition in multicomponent microalloyed austenite. Mater. Lett. 2007, 61, 2227–2230. [Google Scholar] [CrossRef]
- Xiaodong, L.; Solberg, J.K.; Gjengedal, R.; Kluken, A.O. An expression for solubility product of complex carbonitrides in multicomponent microalloyed austenite. Scr. Metall. Mater. 1994, 31, 1607–1612. [Google Scholar] [CrossRef]
Figure 1.
The calculated volume fraction of precipitates considering simple precipitates for: (a) high-Nb steel; (b) low-Nb steel.
Figure 2.
The calculated volume fraction of precipitates considering mutual solubility: (a) high-Nb steel; (b) low-Nb steel.
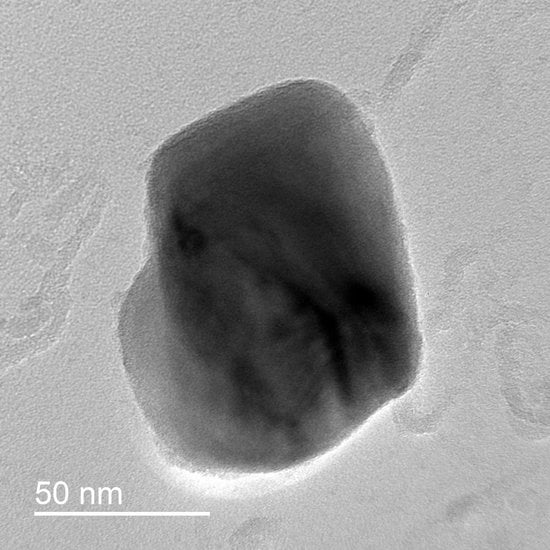
Figure 3.
Transmission electron microscopy (TEM) micrograph examples (a) low magnification Low-Nb Steel austenitized at 1300 °C for 1 h; (b) high magnification from High-Nb Steel austenitized to 1300 °C for 1 h; (c) EDS spectra of particles in (b).
Figure 4.
(a) Average diameter of precipitates at the different austenitization temperatures with soaking time of 1 h and corresponding pinning force; (b) volume fraction and corresponding pinning force. Measurements based on TEM micrography.
Figure 5.
Distribution of particle size: (a,b) Particles smaller than 40 nm measured from ten 20,000× micrographs; (c,d) particles bigger than 40 nm, measured from five 5000× micrographs.
Figure 6.
Experimental volume fraction of precipitates, compared to the calculated expectations: (a) high-Nb steel; (b) low-Nb steel.
Figure 7.
Bright field-Dark field (BF-DF) images and SADP from a precipitate in low-Nb steel austenitized at 1200 °C for 1 h.
Figure 8.
HRTEM, Fast Fourier Transform, and EDS results of a (Ti,Nb)(C,N) precipitate (big) in low-Nb steel austenitized at 1200 °C for 1 h.
Figure 9.
BF–DF and SADP of complex bicrystalline particles found in: (a) low-Nb steel austenitized at 1300 °C for 1 h.; (b) high-Nb steel austenitized to 1150 °C for 1 h.
Figure 10.
Average Feret diameter of Prior-Austenitic Grains. Measured from light microscopy after picral etching.
Figure 11.
Grain size distribution of (a) high-Nb steel; and (b) Low-Nb steel.
Figure 12.
Experimentally determined PAGS compared to calculated predictions: (a) High-Nb steel; and (b) Low-Nb steel.
Figure 13.
Comparison of the reconstructed PAGS maps to a picral etched micrograph. (a) Micrograph after picral etching; (b) EBSD OIM map from quenched specimen; (c) reconstructed OIM map of austenite, based on (b). High-Nb sample austenitized to 1150 °C for 1 h.
Figure 14.
Reconstructed austenite EBSD OIM maps from austenitizing high-Nb steel to: (a) 1150 °C, (b) 1200 °C, (c) 1250 °C, (d) 1300 °C for 1 h; and from austenitizing Low-Nb Steel to: (e) 1150 °C, (f) 1200 °C, (g) 1250 °C and (h) 1300 °C for 1 h. All images are 150 × 190 μm2.
Figure 15.
Distribution of GB misorientation for various temperatures on: (a) High-Nb steel and (b) low-Nb steel.
Table 1.
Alloys used in this study (wt%).
| Alloy | C | Mn | Cu | Ni | Cr | Mo | Nb | V | Ti | N | Al | Si | Fe |
|---|
| High Nb | 0.05 | 1.5 | 0.1 | 0.25 | 0.25 | 0.15 | 0.090 | 0.06 | 0.01 | 0.007 | 0.04 | 0.25 | Base |
| Low Nb | 0.05 | 1.5 | 0.1 | 0.25 | 0.25 | 0.15 | 0.045 | 0.06 | 0.01 | 0.007 | 0.04 | 0.25 | Base |
Table 2.
EDS chemical composition measurements from precipitates in
Figure 2b (at%/wt%).
| Spectra | C | N | Si | Ti | V | Fe | Cu | Nb |
|---|
| Top left | 41.82/20.1 | 32.51/18.2 | 0.15/0.2 | 16.03/30.7 | 0.54/1.1 | 0.1/0.2 | 2.93/7.5 | 5.92/22 |
| Right | 78.7/59.4 | 14.1/12.4 | | 3.97/11.9 | | | 1.37/5.5 | 1.85/10.8 |
| Bottom | 73.5/52 | 17.13/14.1 | | 5.35/15.1 | | | 1.61/6 | 2.34/12.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).