Surface Investigation of Ni81Fe19 Thin Film: Using ARXPS for Thickness Estimation of Oxidation Layers
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. AFM Analysis and Surface Roughness
3.2. Magnetic Properties of NiFe Films
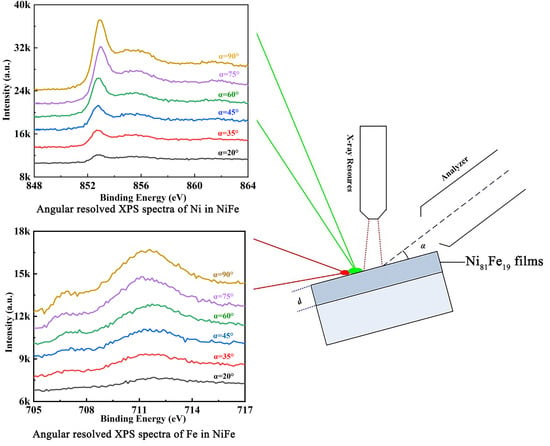
3.3. Oxidation Thickness of NiFe Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Z.; Bennett, S.; Hu, B.; Chen, Y.; Harris, V.G. Magnetic and microwave properties of U-type hexaferrite films with high remanence and low ferromagnetic resonance linewidth. J. Appl. Phys. 2014, 115, 17A504. [Google Scholar] [CrossRef]
- Belyaev, B.A.; Lemberg, K.V.; Serzhantov, A.M.; Leksikov, A.A.; Bal’va, Y.F. Magnetically tunable resonant phase shifters for UHF band. IEEE Trans. Magn. 2015, 51, 1–5. [Google Scholar] [CrossRef]
- Sharma, V.; Khivintsev, Y.; Harward, I.; Kuanr, B.K.; Celinski, Z. Fabrication and characterization of microwave phase shifter in microstrip geometry with Fe film as the frequency tuning element. J. Magn. Magn. Mater. 2019, 489, 165412. [Google Scholar] [CrossRef]
- Kuanr, B.K.; Veerakumar, V.; Camley, R.E.; Celinski, Z. Permalloy (NiFe) nanometer square-antidot arrays: Dynamic modes and use as a monolithic microwave band-pass filter. J. Magn. Magn. Mater. 2019, 484, 272–278. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, B.; Hu, Z.; He, Y.; Hu, T.; Zhao, Y.; Wang, Z.; Zhou, Z.; Cui, W.; Liu, M. Surface roughness evolution induced low secondary electron yield in carbon coated Ag/Al substrates for space microwave devices. Appl. Surf. Sci. 2020, 501, 144236. [Google Scholar] [CrossRef]
- Paiva, D.V.M.; Silva, M.A.S.; Sombra, A.S.B.; Fechine, P.B.A. Properties of the Sr3MoO6 electroceramic for RF/microwave devices. J. Alloys Compd. 2018, 748, 766–773. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, X.; Zhou, Y.; Xie, Y.; Wu, J.; Wang, T.; Chui, S.T.; Xiao, J.Q. Designing and tuning magnetic resonance with exchange interaction. Adv. Mater. 2015, 27, 1351–1355. [Google Scholar] [CrossRef]
- Belmeguenai, M.; Martin, T.; Woltersdorf, G.; Bayreuther, G.; Baltz, V. Microwave spectroscopy with vector network analyzer for interlayer exchange-coupled symmetrical and asymmetrical NiFe/Ru/NiFe. J. Phys. Condens. Matter. 2008, 20, 345206. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, Q.; Xu, J.; Yan, S.; Miao, G.X.; Kang, S.; Dai, Y. Tunable optical mode ferromagnetic resonance in FeCoB/Ru/FeCoB synthetic antiferromagnetic trilayers under uniaxial magnetic anisotropy. Adv. Funct. Mater. 2016, 26, 3738–3744. [Google Scholar] [CrossRef]
- Xu, F.; Liao, Z.; Huang, Q.; Phuoc, N.N.; Ong, C.K.; Li, S. Influence of thickness on magnetic properties and microwave characteristics of NiFe/IrMn/NiFe Trilayers. IEEE Trans. Magn. 2011, 47, 3486–3489. [Google Scholar] [CrossRef]
- Naik, R.; Kota, C.; Payson, J.S.; Dunifer, G.L. Ferromagnetic-resonance studies of epitaxial Ni, Co, and Fe films grown on Cu(100)/Si(100). Phys. Rev. B 1993, 48, 1008–1013. [Google Scholar] [CrossRef]
- Chappert, C.; Le Dang, K.; Beauvillain, P.; Hurdequint, H.; Renard, D. Ferromagnetic resonance studies of very thin cobalt films on a gold substrate. Phys. Rev. B 1986, 34, 3192–3197. [Google Scholar] [CrossRef]
- Biondo, A.; Nascimento, V.P.; Lassri, H.; Passamani, E.C.; Morales, M.A.; Mello, A. Structural and magnetic properties of Ni81Fe19/Zr multilayers. J. Magn. Magn. Mater. 2004, 277, 144–152. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, H.; Teng, J.; Chai, C.; Zhu, F.; Xia, Y.; Chai, X. XPS Studies of magnetic multilayers. J. Univ. Sci. Technol. B 2001, 8, 210–213. [Google Scholar]
- Sakhonenkov, S.S.; Filatova, E.O.; Gaisin, A.U.; Kasatikov, S.A.; Konashuk, A.S. Angle resolved photoelectron spectroscopy as applied to X-ray mirrors: An in-depth study of Mo/Si multilayer systems. Phys. Chem. Chem. Phys. 2019, 21, 25002–250109. [Google Scholar] [CrossRef]
- Zemek, J.; Houdkova, J.; Jiricek, P.; Jelinek, M. Surface and in-depth distribution of sp2 and sp3 coordinated carbon atoms in diamond-like carbon films modified by argon ion beam bombardment during growth. Carbon 2018, 134, 71–79. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Vazquez-Lepe, M.O.; Duong, T.; Arroyave, R. Diffusion of In and Ga in TiN/HfO2/InGaAs nanofilms. J. Appl. Phys. 2013, 114, 143504. [Google Scholar] [CrossRef]
- Dong, B.Z.; Fang, G.J.; Wang, J.F.; Guan, W.J.; Zhao, X.Z. Effect of thickness on structural, electrical, and optical properties of ZnO: Al films deposited by pulsed laser deposition. J. Appl. Phys. 2007, 101, 033713. [Google Scholar] [CrossRef]
- Akhter, M.A.; Mapps, D.J.; Ma Tan, Y.Q.; Petford-Long, A.; Doole, R. Thickness and grain-size dependence of the coercivity in permalloy thin films. J. Appl. Phys. 1997, 81, 4122–4124. [Google Scholar] [CrossRef]
- Berzins, A.; Smits, J.; Petruhins, A. Characterization of Microscopic ferromagnetic defects in thin films using magnetic microscope based on nitrogen-vacancy centres. Mater. Chem. Phys. 2021, 267, 124617. [Google Scholar] [CrossRef]
- John, F.W.; John, W. An Introduction to Surface Analysis by XPS and AES, 1st ed.; East China University of Technology Publisher: Shanghai, China, 2008. [Google Scholar]
- Nagarkar, P.V.; Kulkarni, S.K.; Umbach, E. Surface oxidation investigation of Ni36Fe32Cr14P12B6 glass using angle resolved XPS. Appl. Surf. Sci. 1987, 29, 194–222. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Dupin, J.C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Wang, J.Q. Introduction to Electron Spectroscopy (XPS/XAES/UPS); National Defense Industry Publisher: Beijing, China, 1992. [Google Scholar]
- Cao, Q.X.; Lei, T.M.; Huang, Y.X.; Li, G.F. Fundamentals of Solid Physics; Xidian University Publisher: Xi’an, China, 2008. [Google Scholar]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Carver, J.C.; Schweitzer, G.K.; Carlson, T.A. Use of X-ray photoelectron spectroscopy to study bonding in Cr, Mn, Fe, and Co compounds. J. Chem. Phys. 1972, 57, 973–982. [Google Scholar] [CrossRef]










| Material | ρ (g/cm3) | M (g/mol) | R∞ | Kinetic Energy (eV) |
|---|---|---|---|---|
| Ni | 8.9 | 59 | - | 853.3 |
| NiO | 6.84 | 75 | 0.6 | 853.6 |
| Ni(OH)2 | 4.15 | 93 | 0.3 | 855.0 |
| α (°) | Is(Ni) (%) | Io(NiO) (%) | Io(Ni(OH)2) (%) | R(NiO) = Io(NiO)/Is | R(Ni(OH)2) = Io/Is |
|---|---|---|---|---|---|
| 20 | 20.38 | 46.18 | 33.44 | 2.27 | 1.64 |
| 35 | 32.50 | 37.95 | 29.55 | 1.17 | 0.91 |
| 45 | 36.73 | 35.37 | 27.90 | 0.96 | 0.76 |
| 60 | 40.32 | 33.27 | 26.41 | 0.83 | 0.66 |
| 75 | 42.05 | 32.29 | 25.66 | 0.77 | 0.61 |
| 90 | 42.57 | 32.00 | 25.44 | 0.75 | 0.60 |
| α (°) | Is(Fe) (%) | Io(Fe3O4) (%) | Io(Fe2O3) (%) | R(Fe3O4) = Io(Fe3O4)/Is | R(Fe2O3) = Io(Fe2O3)/Is |
|---|---|---|---|---|---|
| 20 | 2.11 | 7.85 | 90.04 | 3.72 | 42.67 |
| 35 | 8.59 | 8.01 | 83.41 | 0.93 | 9.71 |
| 45 | 12.49 | 7.92 | 79.58 | 0.63 | 6.37 |
| 60 | 16.63 | 7.75 | 75.61 | 0.47 | 4.55 |
| 75 | 18.91 | 7.63 | 73.46 | 0.40 | 3.88 |
| 90 | 19.63 | 7.59 | 72.78 | 0.39 | 3.71 |
| Material | ρ (g/cm3) | M (g/mol) | R∞ | Kinetic Energy (eV) |
|---|---|---|---|---|
| Fe | 7.86 | 56 | - | 710.7 |
| Fe2O3 | 5.24 | 160 | 0.23 | 710.4 |
| Fe3O4 | 5.18 | 232 | 0.16 | 710.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Li, Z.; Jiang, X.; Wu, C.; Liu, Y.; Song, X.; Yu, Z.; Wang, Y.; Lan, Z.; Sun, K. Surface Investigation of Ni81Fe19 Thin Film: Using ARXPS for Thickness Estimation of Oxidation Layers. Metals 2021, 11, 2061. https://doi.org/10.3390/met11122061
He Z, Li Z, Jiang X, Wu C, Liu Y, Song X, Yu Z, Wang Y, Lan Z, Sun K. Surface Investigation of Ni81Fe19 Thin Film: Using ARXPS for Thickness Estimation of Oxidation Layers. Metals. 2021; 11(12):2061. https://doi.org/10.3390/met11122061
Chicago/Turabian StyleHe, Zongsheng, Ziyu Li, Xiaona Jiang, Chuanjian Wu, Yu Liu, Xinglian Song, Zhong Yu, Yifan Wang, Zhongwen Lan, and Ke Sun. 2021. "Surface Investigation of Ni81Fe19 Thin Film: Using ARXPS for Thickness Estimation of Oxidation Layers" Metals 11, no. 12: 2061. https://doi.org/10.3390/met11122061
APA StyleHe, Z., Li, Z., Jiang, X., Wu, C., Liu, Y., Song, X., Yu, Z., Wang, Y., Lan, Z., & Sun, K. (2021). Surface Investigation of Ni81Fe19 Thin Film: Using ARXPS for Thickness Estimation of Oxidation Layers. Metals, 11(12), 2061. https://doi.org/10.3390/met11122061