The Deformation Behavior of Niobium Microalloyed Steel during Lüders Band Formation
Abstract
:1. Introduction
2. Materials and Methods
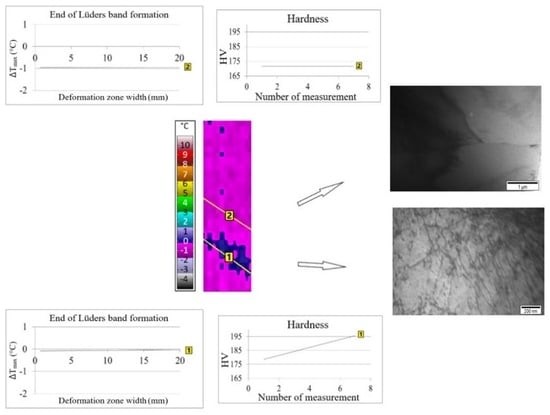
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hallai, J.F.; Kyriakides, S. On the effect of Lüders bands on the bending of steel tubes. Part I: Experiments. Int. J. Solids Struct. 2011, 48, 3275–3284. [Google Scholar] [CrossRef]
- Zhou, T.; Zurob, H.; Zhang, P.; Kuuskman, K.; Cho, S.H.; Burella, D. Control of edge breaks during cold mill processing of commercial and drawing quality low-carbon steels. Ironmak. Steelmak. 2019, 46, 656–662. [Google Scholar] [CrossRef]
- Tsuchida, N.; Tomota, Y.; Nagai, K.; Fukaura, K. A simple relationship between Lüders elongation and work-hardening rate at lower yield stress. Scr. Mater. 2006, 54, 57–60. [Google Scholar] [CrossRef]
- Kamikawa, N.; Huang, X.; Tsuji, N.; Hansen, N. Strengthening mechanisms in nanostructured high-purity aluminium deformed to high strain and annealed. Acta Mater. 2009, 57, 4198–4208. [Google Scholar] [CrossRef]
- Coër, J.; Manach, P.Y.; Laurent, H.; Oliveira, M.C.; Menezes, L.F. Piobert-Lüders plateau and Portevin-Le Chatelier effect in an Al-Mg alloy in simple shear. Mech. Res. Commun. 2013, 48, 1–7. [Google Scholar] [CrossRef]
- Emadoddin, E.; Akbarzadeh, A.; Daneshi, G.H. Correlation between Luder strain and retained austenite in TRIP-assisted cold rolled steel sheets. Mat. Sci. Eng. A 2007, 447, 174–179. [Google Scholar] [CrossRef]
- Saha, R.; Ueji, R.; Tsuji, N. Fully recrystallized nanostructure fabricated without severe plastic deformation in high-Mn austenitic steel. Scr. Mater. 2013, 68, 813–816. [Google Scholar] [CrossRef]
- An, H.; Wu, S.; Zhang, Z.; Figueiredo, R.B.; Gao, N.; Langdon, T.G. Enhanced strength–ductility synergy in nanostructured Cu and Cu–Al alloys processed by high-pressure torsion and subsequent annealing. Scr. Mater. 2012, 66, 227–230. [Google Scholar] [CrossRef]
- Li, Z.; Fu, L.; Fu, B.; Shan, A. Yield point elongation in fine-grained titanium. Mater. Lett. 2013, 96, 1–4. [Google Scholar] [CrossRef]
- Zheng, L.; He, Y.; Moumni, Z. Luders-like band motion and fatigue life of pseudoelastic polycrystalline NiTi shape memory alloy. Scr. Mater. 2016, 123, 46–50. [Google Scholar] [CrossRef]
- Barannikova, S.; Li, Y. Kinetics of deformation bands in a low-carbon steel—Stainless steel bimetal. Metalurgija 2021, 60, 59–62. [Google Scholar] [CrossRef]
- Torkamani, H.; Raygan, S.; Garcia Mateo, C.; Rassizadehghani, J.; Palizdar, Y.; San-Martin, D. Tensile Behavior of Normalized Low Carbon Nb-microalloyed Steel in the Presence of Rare Earth Elements. Mat. Sci. Eng. A 2019, 749, 56–64. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, F.; Ma, H. Structural Stability Analysis of Steel Tubular Scaffold with Couplers Based on Direct Analysis Method. Teh. Vjesn. 2022, 29, 408–414. [Google Scholar]
- Cumin, J.; Novoselović, D.; Samardžić, M.; Samardžić, I. Statistical analysis of different mathematical models for stress-strain curves of AISI 321 stainless steel. Metalurgija 2023, 62, 391–393. [Google Scholar]
- Dziubińska, A.; Surdacki, P. Numerical Analysis of the New Forming Process of the Aircraft Bracket Forging Made of AZ91 Alloy at Different Rates of Deformation. Teh. Vjesn. 2022, 29, 634–640. [Google Scholar]
- Romanova, V.; Balokhonov, R.; Zinovieva, O. Mesoscale deformation-induced surface phenomena in loaded polycrystals. FU Mech. Eng. 2021, 19, 187–198. [Google Scholar] [CrossRef]
- Qiu, H.; Inoue, T.; Ueji, R. In-Situ Observation of Lüders Band Formation in Hot-Rolled Steel via Digital Image Correlation. Metals 2020, 10, 530. [Google Scholar] [CrossRef]
- Spišák, E.; Majerníková, J.; Kaščák, Ľ.; Slota, J. Influence of plastic deformation inhomogeneity on corrosion resistance of tin plates. Metalurgija 2021, 60, 67–70. [Google Scholar]
- Danilov, V.I.; Orlova, D.V.; Gorbatenko, V.V.; Danilova, L.V. Effect of Temperature on the Kinetics of Localized Plasticity Autowaves in Lüders Deformation. Metals 2023, 13, 773. [Google Scholar] [CrossRef]
- Samardzic, I.; Vuherer, T.; Maric, D.; Konjatic, P. Influence of vibrations on residual stresses distribution in welded joints. Metalurgija 2015, 54, 527–530. [Google Scholar]
- Brlić, T.; Rešković, S.; Jurković, Z.; Janeš, G. Mathematical modeling of influence parameters during formation and propagation of the Lüders bands. FU Mech. Eng. 2020, 18, 595–610. [Google Scholar] [CrossRef]
- Srinivasan, N.; Narayanaswamy, R.; Venkatraman, B. Advanced imaging for early prediction and characterization of zone of Lüders band nucleation associated with pre-yield microstrain. Mat. Sci. Eng. A 2013, 561, 203–211. [Google Scholar]
- Cottrell, H.; Bilby, B.A. Dislocation Theory of Yielding and Strain Ageing of Iron. Proc. Phys. Soc. A 1949, 62, 49–62. [Google Scholar] [CrossRef]
- Beardsmore, D.W.; Quinta da Fonseca, J.; Romero, J.; English, C.A.; Ortner, S.R.; Sharples, J.; Sherry, A.H.; Wilkes, M.A. Study of Lüders phenomena in reactor pressure vessel steels. Mat. Sci. Eng. A 2013, 588, 151–166. [Google Scholar] [CrossRef]
- Barišić, B. Analiza Pojave Lüdersovih Traka u Procesu Izrade Proizvoda iz Tankostjenog Lima. Ph.D. Thesis, University of Rijeka, Rijeka, Croatia, 2005. [Google Scholar]
- Qiu, H.; Inoue, T.; Ueji, R. Experimental measurement of the variables of Lüders deformation in hot-rolled steel via digital image correlation. Mater. Sci. Eng. A 2020, 790, 139756. [Google Scholar] [CrossRef]
- Polukhina, O.N.; Vichuzhanin, D.I.; Khotinov, V.A.; Schapov, G.V.; Farber, V.M. Luders Deformation Mechanisms on Yield Point in X80 Grade Pipeline with Ultrafine Structure. In Proceedings of the XIX International Scientific-Technical Conference “The Ural School-Seminar of Metal Scientists-Young Researchers”, Yekaterinburg, Russia, 19–23 November 2018; KnE Engineering: Yekaterinburg, Russia, 2019. [Google Scholar]
- Qiu, H.; Ueji, R.; Inoue, T. Yield-Point Phenomenon and Plastic Bands in Ferrite-Pearlite Steels. Materials 2022, 16, 195. [Google Scholar] [CrossRef]
- Hickey, J.L.R.; Rouland, S.; Britton, T.B. Heterogeneous local plastic deformation of interstitial free steel revealed using in-situ tensile testing and high angular resolution electron backscatter diffraction. arXiv 2018, arXiv:1807.02017. [Google Scholar]
- Hultgren, A. The Morphology of Lüders Deformation in Polycristals. Scand. J. Metall. 1972, 1, 17–22. [Google Scholar]
- Gao, S.; Bai, Y.; Zheng, R.; Tian, Y.; Mao, W.; Shibata, A.; Tsuji, N. Mechanism of huge Lüders-type deformation in ultrafine grained austenitic stainless steel. Scr. Mater. 2019, 159, 28–32. [Google Scholar] [CrossRef]
- Rešković, S.; Slokar Benić, L.; Lovrenić-Jugović, M. The Interdependence of the Degree of Precipitation and Dislocation Density during the Thermomechanical Treatment of Microalloyed Niobium Steel. Metals 2020, 10, 294. [Google Scholar] [CrossRef]
- Brlić, T.; Rešković, S.; Jandrlić, I. Influence of Niobium Content on Strain Amount in Lüders Bands in Niobium Microalloyed Steel. Met. Mater. Int. 2020, 26, 179–187. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Z.; Liu, X.; Wang, G. Effect of microcontent Nb in solution on the strength of low carbon steels. Mat. Sci. Eng. A 2004, 379, 384–390. [Google Scholar] [CrossRef]
- Slokar Benić, L.; Jandrlić, I.; Rešković, S. Effect of subsequent heating on the microstructure and mechanical properties of Nb microalloyed steel. In Proceedings of the 18th International Foundrymen Conference, Coexistence of Material Science and Sustainable Technology in Economic Growth, Sisak, Croatia, 15–17 May 2019; pp. 180–187. [Google Scholar]






| Element, wt% | C | Mn | Si | P | S | Al | Nb | N |
|---|---|---|---|---|---|---|---|---|
| Microalloyed steel | 0.12 | 0.78 | 0.18 | 0.011 | 0.018 | 0.020 | 0.048 | 0.0080 |
| Sample | Deformation (H-W) ε/Ǻ |
|---|---|
| Sample without Lüders band | 0.00170 ± 0.00094 |
| Sample with Lüders band | 0.00205 ± 0.00133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brlić, T.; Rodič, T.; Samardžić, I.; Marciuš, M.; Matvija, M.; Rešković, S. The Deformation Behavior of Niobium Microalloyed Steel during Lüders Band Formation. Metals 2023, 13, 1678. https://doi.org/10.3390/met13101678
Brlić T, Rodič T, Samardžić I, Marciuš M, Matvija M, Rešković S. The Deformation Behavior of Niobium Microalloyed Steel during Lüders Band Formation. Metals. 2023; 13(10):1678. https://doi.org/10.3390/met13101678
Chicago/Turabian StyleBrlić, Tin, Tomaž Rodič, Ivan Samardžić, Marijan Marciuš, Miloš Matvija, and Stoja Rešković. 2023. "The Deformation Behavior of Niobium Microalloyed Steel during Lüders Band Formation" Metals 13, no. 10: 1678. https://doi.org/10.3390/met13101678
APA StyleBrlić, T., Rodič, T., Samardžić, I., Marciuš, M., Matvija, M., & Rešković, S. (2023). The Deformation Behavior of Niobium Microalloyed Steel during Lüders Band Formation. Metals, 13(10), 1678. https://doi.org/10.3390/met13101678