In our project we propose treating anodic lodes and scrapings from the zinc electrolysis. It would be also possible to recover other raw materials/residues of manganese, classified in: chemically refractories (difficultly lixiviated, such as slags, powders, or other residues coming from the pyrometallurgy of manganese) and reactive (easily lixiviated, such as anodic lodes and scrapings of the zinc electrolysis and manganese carbonates). We designed a process that we have divided into four sub-processes (see
Figure 1): acid leaching at low temperature (50 °C), acid leaching at high temperature (150–200 °C), neutralization with CaO (s), and chemical purification and electrolysis process. In this way, a conceptual design of plant was carried out while considering a production of 1000 kg of Mn (metal) every day. Even when the objective is obtaining electrolytic manganese, widely used in the production of aluminum and copper alloys, for special grades of stainless steel and other special steels, and for electronic applications [
13], the process described could be applied for the obtaining of manganese sulphate, which could be sold as MnSO
4 or used in the production of manganese oxide (IV) and manganese oxide (II).
The basic characteristics of the process for obtaining manganese aqueous solutions used in the manufacture of electrolytic manganese are:
As it was previously mentioned, the proposed industrial process was divided into four stages or sub-processes. We will describe each one of the stages separately, being each of them supported by laboratory trials.
2.2.1. Sulphation at Low Temperature (50 °C)
The objective of this stage of the process is reducing MnO
2 in presence of a reductant agent, SO
2 (
g) (other reducing reagents have been used in treating manganese ores, such as oxalic acid, hydrogen peroxide and glucose [
15], cornstalk [
16], phenols [
17], cane molasses [
19], CaS [
20], carbon [
23] or waste tea [
34]) and obtaining a solution of MnSO
4 (
aq) to be sent to the neutralization stage, and then to electrolysis. The mixed leaching allows for recovering almost all of the manganese of the anodic lodes and scrapings (95% is supposed in the calculations). The reactivity of the waste is exploited in this stage as a kind of neutralization of the sulfuric acid coming from other stages, supported by the utilization of SO
2 as reductant reagent. The low manganese waste contains valuable elements, such as lead and silver (see
Table 2), coming from both the low and high temperature leaching processes.
The laboratory scale process begins with the drying of the lodes and scrapings in stove at 110 °C, and homogenization of the by-product. It was then mixed with Na
2SO
3, H
2SO
4 and water to obtain a solution containing manganese as sulphate and impurities, and leaving a solid product that contained lead sulphate and impurities (
Table 2). The presence of Na
2SO
3 (
s) allows for the generation of SO
2 (
g) that acts as reductant agent of the by-product.
Tests were carried out in a hastelloy reactor (Laboratorio de Metalurgia, Dpto. de Ciencia de los Materiales e Ingeniería Metalúrgica, Universidad de Oviedo, Oviedo (Asturias), Spain) with different entries, allowing the introduction of a thermocouple to control the temperature and the feeding of the reagents. Besides, the reactor has an agitation system to homogenize and mix the different reagents. As the reaction of decomposition of the Na2SO3 (s) to generate SO2 (g) is exothermic, the reactor is insulated with refractory wool with the purpose of minimizing the heat loss (this heat is used to make more favorable the process). In the proposed industrial process, the SO2 (g) can be supplied directly as gas instead of using Na2SO3 (s) in the decomposition of H2SO4 (aq) to obtain SO2 (g) used as reducing agent.
Once the amounts of the different reagents previously mentioned were weighted, they were loaded in the reactor. The feeding process had a sequence that was: with the reactor open the Na
2SO
3 (
s) was previously loaded with the manganese by-product and 2/3 of the water; the reactor is then closed and the agitation system is connected (at low agitation speed, around 30 rpm), while the H
2SO
4 (
aq) is loaded into the vessel in small amounts; finally, 1/3 of the water is loaded into the reactor, it is completely closed and from this moment and the process has a duration of 30 min. A thermocouple was used to control the temperature, and it is observed that it was kept at 40–60 °C because of the exothermic reaction previously mentioned. Once the process finished, the solution is filtrated and an aqueous solution of MnSO
4 and a solid by-product, containing PbSO
4 (lead sulphate is poorly soluble in water) and other impurities, are obtained, being the solution analyzed by atomic absorption spectroscopy to evaluate the amount of manganese in the solution. The by-product coming from the filtration process was analyzed by using X-ray fluorescence and the results are shown in
Table 2. Lead, as well as manganese and zinc, appear in this by-product as sulphate. This by-product (RA in
Figure 1) contains significant amounts of lead and silver that could be treated with the purpose of recovering both of them (see pages 378–404 in [
9]), thus making the process much more economically profitable.
The amounts of reagent in this process at low temperature were calculated by means of the following chemical reaction considering 15 g. of anodic lode as base of calculus:
This reaction is thermodynamically favorable (
), according to the software HS5.1 (Outokumpu Research Oy, 5.11, Pori, Finland) even at room temperature. However, the real process includes other reactions of importance like that one:
As
is unstable, it decomposes and:
And the simplified reaction for the reduction of the MnO
2 in the anodic lode is:
Being the
the reductant agent used in the process (see Equation (5)). For that reason, when the amount of reductant agents was calculated (verifying the last equations) the amount of water and lode were kept constant. In this way, anodic lode and scrapings were a constant value of 15 g. To evaluate the effect of milling two situations were considered after 30 s milling and after 90 s milling (a finer granulometry will increase the leaching, but also a product with a more homogenous granulometry is obtained as lodes and scrapings have different initial granulometries). The results are shown in
Table 3 and
Table 4.
It should be considered that the manual introduction of H
2SO
4 and the nearly simultaneous generation of SO
2 (
g) cause the release of this gas, and consequently the loss of reducing gas. If the supply of H
2SO
4 had carried out automatically, then the manganese extraction would be better. Moreover, the direct supply of SO
2 (
g) would also increase the manganese extraction. This is the reason of a lower recovery in Condition 2 (in the industrial scale process the SO
2 supply will be automatic). It is also significant the improvement in the manganese extraction when the higher the milling, as the lower the particle size the easier the chemical lixiviation as the surface is increased and the reactions solid-gas became more favorable [
11].
The residue obtained after filtering was analyzed by X-ray diffraction (PANalytical X’Pert Powder, Servicios Científico-Técnicos, Universidad de Oviedo, Oviedo (Asturias), Spain) and the following phases were obtained (see
Figure 2, which is consistent with the information provided in
Table 2): anglesite (PbSO
4) as the main phase, and sulphates of manganese and strontium. Lead is recovered in the solid residue obtained after the filtration as lead sulphate. This lead sulphate could be used in the manufacture of lead [
9]. The presence of certain amount of manganese in the solid residue is always unavoidable, as we did not reach a full extraction of manganese. Other impurities also end in the solid residue as calcium, strontium, and potassium, but also silver. The presence of silver and lead in the solid residue of filtration makes it economically interesting. The solution containing most of the manganese (as MnSO
4) should be purified before being used to produce electrolytic manganese.
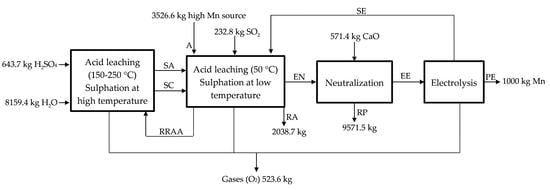
The acid leaching/sulphation at low temperature (50 °C, temperature reached because of the exothermic reactions) is described in
Figure 3 for the industrial scale process. The by-product/waste is loaded in a reactor (A, high MnO
2 source), where it is leached with SO
2 (
g) (like that proposed in a laboratory scale but replacing Na
2SO
3 (
s), used in the generation of SO
2 (
g), by using directly SO
2 (g)) (see Equation (5)) and H
2SO
4 (
aq) (see Equation (7)). MnO
2 is reduced in presence of both SO
2 (g) (20% of the initial manganese, according to Equation (5)) and H
2SO
4 (
aq) (10% of the initial according to Equation (7)) from
to
, and a solution of MnSO
4 (
aq) is obtained (a neutralization also happens in this stage as H
2SO
4 (
aq) is used in the lixiviation of the manganese source). The presence of a reducing agent is always necessary for the complete extraction of the manganese, for instance, in the process described by Sanchez-Recio and Sancho [
23], they take advantage of the presence of carbon in the powders used as manganese source [
23]. The acid solution of MnSO
4 (aq) is sent with the MnSO
4 (
aq) solution obtained in the acid leaching at high temperature (SC, hot sulfated) to neutralization (EN, entrance to neutralization). 70% of the initial manganese is treated in the acid leaching at high temperature (RRAA, recycled residue of acid leaching). The manganese source (anodic lodes and scrapings) contains significant amounts of impurities (lead, strontium, silver, etc.) that leave the process as a product, which we call RA (Residue low MnO
2). Most of the impurities enter and leave the process as sulphate so there are not H
2SO
4 (
aq) losses, but ~3.5% Mn is lost in this residue that leaves the process wet.
Figure 3 describes the acid leaching at low temperature.