The Effect of Cerium Ions on the Structure, Porosity and Electrochemical Properties of Si/Zr-Based Hybrid Sol-Gel Coatings Deposited on Aluminum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of the Sols
2.3. Sample Preparation
2.4. Sample Characterization
2.5. Corrosion Evaluation
3. Results
3.1. Composition and Structure of Hybrid Coatings
3.2. Thermal Properties
3.3. Electrochemical Characterization
3.3.1. DC Electrochemical Tests
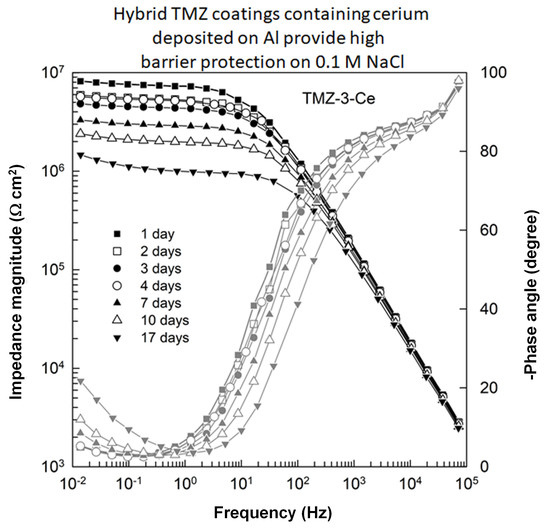
3.3.2. Long-Term Corrosion Tests
4. Conclusions
- Hybrid Si/Zr sol-gel coatings with three different contents of Zr (0.06, 0.12 and 0.48 mol) without and with the addition of Ce3+ ions (0.5 wt. % Ce(NO3)3) were synthesized for the protection of aluminum against corrosion in 0.1 M NaCl. These sol-gel coatings were denoted TMZ-1, TMZ-2, TMZ-3, TMZ-1-Ce, TMZ-2-Ce and TMZ-3-Ce, respectively.
- According to the results of Raman spectroscopy, photothermal beam deflection spectroscopy and electrochemical techniques, TMZ-3 and Ce-doped TMZ-3-Ce coatings, containing the highest Zr content, exhibit structural homogeneity and decreased porosity. Hence, excellent protecting properties and corrosion resistance were imparted to the underlying aluminum substrate. Corrosion current density, jcorr, decreased for two orders of magnitude in comparison to the uncoated Al substrate (see Table 2).
- The beneficial effect of the Ce-doping on the coating’s protection properties was more pronounced upon long-lasting immersion of coated aluminum samples in 0.1 M NaCl. The excellent barrier properties and long-lasting anticorrosive protection properties of Ce-doped coatings were related to the combined effect of a more condensed Si−O−Zr network structure and the passivating/inhibiting effect of Ce3+ ions. The synergism between Zr and Ce ions integrated in the coating structure results in a simultaneous dual action of Ce ions: in the coating’s self-healing process as inhibiting agents and in producing an effective anticorrosive barrier to the underlying Al substrate.
List of Abbreviations
| AA7075-T6 | aluminum alloy 7075-T6 |
| AFM | atomic force microscopy |
| CNLS | the complex non-linear least squares fit analysis software |
| CPE | constant phase element |
| DLS | dynamic light scattering |
| EEC | electric equivalent circuit |
| EIS | electrochemical impedance spectroscopy |
| EDS | energy dispersive X-ray spectrometry |
| FTIR | Fourier transform infrared spectroscopy |
| GDOES | glow discharge optimal emission spectroscopy |
| MAA | methacrylic acid, H2C=C(CH3)COOH |
| MAPTMS | 3-methacryloxypropyltrimethoxy silane, H2C=C(CH3)CO2(CH2)3Si(OCH3)3 |
| NMR | nuclear magnetic resonance spectroscopy |
| PBD | photothermal beam deflection spectroscopy |
| SEM | scanning electron spectroscopy |
| TEOS | tetraethyl orthosilicate, Si(OC2H5)4 |
| TG | temperature gradient |
| TGA | thermogravimetric analysis |
| TMZ | Si/Zr-based hybrid sol-gel coatings |
| TMZ-Ce | Ce-doped Si/Zr-based hybrid sol-gel coatings |
| ZTP | zirconium tetrapropoxide, Zr(OCH2CH2CH3)4 |
List of Symbols
| D0 | thermal diffusivity of material without cavities (m2/s) |
| Ds | thermal diffusivity of porous material (m2/s) |
| dE/dt | potential scan rate (V·s−1) |
| E | potential (V) |
| Ecorr | corrosion potential (V) |
| Eoc | open circuit potential (V) |
| f | frequency (Hz) |
| j | current density (A·cm–2) |
| jcorr | corrosion current density (A·cm–2) |
| jω | complex variable for sinusoidal perturbations with ω = 2πf |
| k0 | thermal conductivity of material without cavities (W/(m·K)) |
| ks | thermal conductivity of porous material (W/(m·K)) |
| n | exponent of the constant phase element |
| ncoat | exponent of the constant phase element related to the coating |
| ndl | exponent of the constant phase element related to the double layer capacitance |
| noxide | exponent of the constant phase element related to the intermediate oxide layer |
| P | coating porosity (%) |
| Q | frequency-independent real constant (Ω−1·cm–2 sn) |
| Qcoat | pseudo-capacitance of the film|electrolyte interface (Ω−1·cm–2·sn) |
| Qdl | double layer interfacial capacitance (Ω−1·cm–2·sn) |
| Qoxide | pseudo-capacitance of the intermediate oxide layer (Ω−1·cm–2·sn) |
| R | resistance (Ω·cm2) |
| Rcoat | coating resistance (Ω·cm2) |
| Rct | charge-transfer resistance describing the Faradaic reaction at the metal|electrolyte solution interface (Ω·cm2) |
| Roxide | resistance of the intermediate oxide layer (Ω·cm2) |
| Rp | polarization resistance (Ω·cm2) |
| RΩ | ohmic resistance (Ω·cm2) |
| Z | impedance (Ω·cm2) |
| |Z| | impedance magnitude (Ω·cm2) |
| Greek Letters | |
| γ | ratio of thermal conductivity of cavities to material |
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A


References
- Vargel, C. Corrosion of Aluminium, 2nd ed.; Elsevier: Kidlington, UK, 2004; ISBN 0 08 044495 4. [Google Scholar]
- Zhao, J.; Xia, L.; Sehgal, A.; Lu, D.; McCreery, R.L.; Frankel, G.S. Effects of chromate and chromate conversion coatings on corrosion of aluminum alloy 2024-T3. Surf. Coat. Technol. 2001, 140, 51–57. [Google Scholar] [CrossRef]
- Kendig, M.; Jeanjaquet, S.; Addison, R.; Waldrop, J. Role of hexavalent chromium in the inhibition of corrosion of aluminum alloys. Surf. Coat. Technol. 2001, 140, 58–66. [Google Scholar] [CrossRef]
- ECHA European Chemicals Agency. ANNEX XVII TO REACH—Conditions of Restrictions. Entry 47—Chromium VI compounds. Available online: https://echa.europa.eu/documents/10162/1f775bd4-b1b0-4847-937f-d6a37e2c0c98 (accessed on 4 April 2018).
- VOC Solvents Emissions Directive—Environment—European Commission. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31999L0013 (accessed on 7 November 2017).
- Forsyth, M.; Seter, M.; Tan, M.Y.; Hinton, B. Recent developments in corrosion inhibitors based on rare earth metal compounds. Corros. Eng. Sci. Technol. 2014, 49, 130–135. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Abrahami, S.T.; de Kok, J.M.M.; Terryn, H.; Mol, J.M.C. Towards Cr(VI)-free anodization of aluminum alloys for aerospace adhesive bonding applications: A review. Front. Chem. Sci. Eng. 2017, 11, 465–482. [Google Scholar] [CrossRef]
- Arkles, B. Commercial Applications of Sol-Gel-Derived Hybrid Materials. MRS Bull. 2001, 26, 402–408. [Google Scholar] [CrossRef]
- Han, Y.-H.; Taylor, A.; Knowles, K.M. Scratch resistance and adherence of novel organic–inorganic hybrid coatings on metallic and non-metallic substrates. Surf. Coat. Technol. 2009, 203, 2871–2877. [Google Scholar] [CrossRef]
- Andreeva, D.V.; Shchukin, D.G. Smart self-repairing protective coatings. Mater. Today 2008, 11, 24–30. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I.; Lekka, M.; Andreatta, F.; Fedrizzi, L. Corrosion behaviour and chemical stability of transparent hybrid sol-gel coatings deposited on aluminium in acidic and alkaline solutions. Prog. Org. Coat. Available online: https://doi.org/10.1016/j.porgcoat.2018.02.025 (accessed on 22 March 2018).
- Castro, Y.; Aparicio, M.; Moreno, R.; Duran, A. Silica-zirconia alkali-resistant coatings by sol-gel route. J. Sol-Gel Sci. Technol. 2005, 35, 41–50. [Google Scholar] [CrossRef]
- Andreatta, F.; Paussa, L.; Lanzutti, A.; Rosero Navarro, N.C.; Aparicio, M.; Castro, Y.; Duran, A.; Ondratschek, D.; Fedrizzi, L. Development and industrial scale-up of ZrO2 coatings and hybrid organic–inorganic coatings used as pre-treatments before painting aluminium alloys. Prog. Org. Coat. 2011, 72, 3–14. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Zhang, Y.; Liu, L.; Xu, L.; Liu, Y.; Zhang, L.; Luo, Z.; Cen, K. Effect of zirconium(IV) propoxide concentration on the thermophysical properties of hybrid organic-inorganic films. J. Appl. Phys. 2008, 104, 013528. [Google Scholar] [CrossRef]
- Rodič, P.; Iskra, J.; Milošev, I. A hybrid organic–inorganic sol–gel coating for protecting aluminium alloy 7075-T6 against corrosion in Harrison’s solution. J. Sol-Gel Sci. Technol. 2014, 70, 90–103. [Google Scholar] [CrossRef]
- Rodič, P.; Iskra, J.; Milošev, I. Study of a sol–gel process in the preparation of hybrid coatings for corrosion protection using FTIR and 1H NMR methods. J. Non-Cryst. Solids 2014, 396–397, 25–35. [Google Scholar] [CrossRef]
- Rodič, P.; Mertelj, A.; Borovšak, M.; Benčan, A.; Mihailović, D.; Malič, B.; Milošev, I. Composition, structure and morphology of hybrid acrylate-based sol–gel coatings containing Si and Zr composed for protective applications. Surf. Coat. Technol. 2016, 286, 388–396. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. Corrosion Properties of UV Cured Hybrid Sol-Gel Coatings on AA7075-T6 Determined under Simulated Aircraft Conditions. J. Electrochem. Soc. 2014, 161, C412–C420. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. Electrochemical and Salt Spray Testing of Hybrid Coatings Based on Si and Zr Deposited on Aluminum and Its Alloys. J. Electrochem. Soc. 2015, 162, C592–C600. [Google Scholar] [CrossRef]
- Varma, P.C.R.; Colreavy, J.; Cassidy, J.; Oubaha, M.; Duffy, B.; McDonagh, C. Effect of organic chelates on the performance of hybrid sol–gel coated AA 2024-T3 aluminium alloys. Prog. Org. Coat. 2009, 66, 406–411. [Google Scholar] [CrossRef]
- Varma, P.C.R.; Colreavy, J.; Cassidy, J.; Oubaha, M.; McDonagh, C.; Duffy, B. Corrosion Protection of AA 2024-T3 Aluminium Alloys Using 3, 4-Diaminobenzoic Acid Chelated Zirconium-Silane Hybrid Sol-Gels. Thin Solid Films 2010, 518, 5753–5761. [Google Scholar] [CrossRef]
- Varma, P.C.R.; Cassidy, J.; Oubaha, M.; McDonagh, C.; Colreavy, J.; Duffy, B. Corrosion Protection Properties of Various Ligand Modified Organic Inorganic Hybrid Coating on AA 2024-T3. ECS Trans. 2010, 24, 231–246. [Google Scholar] [CrossRef]
- Paussa, L.; Navarro, N.C.R.; Bravin, D.; Andreatta, F.; Lanzutti, A.; Aparicio, M.; Durán, A.; Fedrizzi, L. ZrO2 sol–gel pre-treatments doped with cerium nitrate for the corrosion protection of AA6060. Prog. Org. Coat. 2012, 2, 311–319. [Google Scholar] [CrossRef]
- Handbook of Smart Coatings for Materials Protection; Makhlouf, A.S.H. (Ed.) Woodhead Publishing Ltd.: Cambridge, UK, 2014; ISBN 0-85709-680-X. [Google Scholar]
- Rosero-Navarro, N.C.; Figiel, P.; Jedrzejewski, R.; Biedunkiewicz, A.; Castro, Y.; Aparicio, M.; Pellice, S.A.; Durán, A. Influence of cerium concentration on the structure and properties of silica-methacrylate sol–gel coatings. J. Sol-Gel Sci. Technol. 2010, 54, 301–311. [Google Scholar] [CrossRef]
- Druart, M.-E.; Recloux, I.; Thai, T.T.; Ershov, S.; Snyders, R.; Olivier, M.-G. Impact of the addition of cerium salts (Ce(III) and Ce(IV)) on formation and ageing of a silica sol-gel layer. Surf. Coat. Technol. 2016, 304, 40–50. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Aruna, S.T.; Anandan, C.; Bera, P.; Sampath, S. EIS and XPS studies on the self-healing properties of Ce-modified silica-alumina hybrid coatings: Evidence for Ce(III) migration. Surf. Coat. Technol. 2017, 309, 363–370. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. Corrosion Inhibition of Pure Aluminium and Alloys AA2024-T3 and AA7075-T6 by Cerium(III) and Cerium(IV) Salts. J. Electrochem. Soc. 2016, 163, C85–C93. [Google Scholar] [CrossRef]
- Milošev, I.; Rodič, P. Cerium chloride and acetate salts as corrosion inhibitors for aluminium alloy AA7075-T6 in sodium chloride solution. Corrosion 2016, 72, 1021–1034. [Google Scholar] [CrossRef]
- Volarič, B.; Milošev, I. Rare earth chloride and nitrate salts as individual and mixed inhibitors for aluminium alloy 7075-T6 in chloride solution. Corros. Eng. Sci. Technol. 2017, 52, 201–211. [Google Scholar] [CrossRef]
- Andreatta, F.; Lohrengel, M.M.; Terryn, H.; de Wit, J.H.W. Electrochemical characterisation of aluminium AA7075-T6 and solution heat treated AA7075 using a micro-capillary cell. Electrochimica Acta 2003, 48, 3239–3247. [Google Scholar] [CrossRef]
- Andreatta, F.; Druart, M.-E.; Lanzutti, A.; Lekka, M.; Cossement, D.; Olivier, M.-G.; Fedrizzi, L. Localized corrosion inhibition by cerium species on clad AA2024 aluminium alloy investigated by means of electrochemical micro-cell. Corros. Sci. 2012, 65, 376–386. [Google Scholar] [CrossRef]
- Paussa, L.; Andreatta, F.; De Felicis, D.; Bemporad, E.; Fedrizzi, L. Investigation of AA2024-T3 surfaces modified by cerium compounds: A localized approach. Corros. Sci. 2014, 78, 215–222. [Google Scholar] [CrossRef]
- Fontinha, I.R.; Salta, M.M.; Zheludkevich, M.L.; Ferreira, M.G.S.; Figueira, R.B.; Pereira, E.V.; Silva, C.J.R. Influence of pH on the Corrosion Protection of Epoxy-Silica-Zirconia Sol-Gel Coatings Applied on EN AW-6063 Aluminium Alloy. ECS Trans. 2014, 58, 9–16. [Google Scholar] [CrossRef]
- Fedel, M.; Callone, E.; Fabbian, M.; Deflorian, F.; Dirè, S. Influence of Ce3+ doping on molecular organization of Si-based organic/inorganic sol-gel layers for corrosion protection. Appl. Surf. Sci. 2017, 414, 82–91. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Liu, J.; Li, S.; Xue, B.; Zhang, Y.; Yin, X. Effects of cerium salts on corrosion behaviors of Si–Zr hybrid sol–gel coatings. Chin. J. Aeronaut. 2015, 28, 600–608. [Google Scholar] [CrossRef]
- Cambon, J.-B.; Esteban, J.; Ansart, F.; Bonino, J.-P.; Turq, V.; Santagneli, S.H.; Santilli, C.V.; Pulcinelli, S.H. Effect of cerium on structure modifications of a hybrid sol–gel coating, its mechanical properties and anti-corrosion behavior. Mater. Res. Bull. 2012, 47, 3170–3176. [Google Scholar] [CrossRef] [Green Version]
- Korte, D.; Franko, M. Application of complex geometrical optics to determination of thermal, transport, and optical parameters of thin films by the photothermal beam deflection technique. J. Opt. Soc. Am. A 2015, 32, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Pal, S.; Ludwig, A.; Wieck, A.D. On the infrared absorption coefficient measurement of thick heavily Zn doped GaAs using spectrally resolved modulated photothermal infrared radiometry. J. Appl. Phys. 2017, 122, 229901. [Google Scholar] [CrossRef]
- Chrobak, Ł.; Maliński, M.; Pawlak, M. Measurements of the optical absorption coefficient of Ar8+ ion implanted silicon layers using the photothermal radiometry and the modulated free carrier absorption methods. Infrared Phys. Technol. 2014, 67, 604–608. [Google Scholar] [CrossRef]
- Maliński, M.; Chrobak, Ł. Numerical analysis of absorption and transmission photoacoustic spectra of silicon samples with differently treated surfaces. Opto-Electron. Rev. 2011, 19, 46–50. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochimica Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Lasia, A. Electrochemical Impedance Spectroscopy and its Applications. In Modern Aspects of Electrochemistry; Conway, B.E., Bockris, J.O., White, R.E., Eds.; Springer: Boston, MA, USA, 2002; Volume 32, pp. 143–248. ISBN 978-0-306-45964-1. [Google Scholar]
- McMillan, P. Structural studies of silicate glasses and melts-applications and limitations of Raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Kalampounias, A.G. IR and Raman spectroscopic studies of sol–gel derived alkaline-earth silicate glasses. Bull. Mater. Sci. 2011, 34, 299–303. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Salvado, I.M.M.; Ferreira, M.G.S. Corrosion protective properties of nanostructured sol–gel hybrid coatings to AA2024-T3. Surf. Coat. Technol. 2006, 200, 3084–3094. [Google Scholar] [CrossRef]
- Araújo, V.D.; Avansi, W.; de Carvalho, H.B.; Moreira, M.L.; Longo, E.; Ribeiro, C.; Bernardi, M.I.B. CeO2 nanoparticles synthesized by a microwave-assisted hydrothermal method: evolution from nanospheres to nanorods. CrystEngComm 2012, 14, 1150–1154. [Google Scholar] [CrossRef]
- Silva, I.d.C.; Sigoli, F.A.; Mazali, I.O. Reversible Oxygen Vacancy Generation on Pure CeO2 Nanorods Evaluated by in Situ Raman Spectroscopy. J. Phys. Chem. C 2017, 121, 12928–12935. [Google Scholar] [CrossRef]
- Assefa, Z.; Haire, R.G.; Caulder, D.L.; Shuh, D.K. Correlation of the oxidation state of cerium in sol-gel glasses as a function of thermal treatment via optical spectroscopy and XANES studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Hinton, B.R.W.; Arnott, D.R.; Ryan, N.E. The inhibition of aluminium alloy corrosion by cerous cations. Met. Forum 1984, 7, 211–217. [Google Scholar]
- Abdolah Zadeh, M.; van der Zwaag, S.; Garcia, S.J. Routes to extrinsic and intrinsic self-healing corrosion protective sol-gel coatings: A review. Self-Heal. Mater. 2013, 1, 1–18. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization Resistance Method for Determination of Instantaneous Corrosion Rates. Corrosion 2000, 56, 199–218. [Google Scholar] [CrossRef]
- Mansfeld, F. Fundamental aspects of the polarization resistance technique—the early days. J. Solid State Electrochem. 2009, 13, 515–520. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Yasakau, K.A.; Bastos, A.C.; Karavai, O.V.; Ferreira, M.G.S. On the application of electrochemical impedance spectroscopy to study the self-healing properties of protective coatings. Electrochem. Commun. 2007, 9, 2622–2628. [Google Scholar] [CrossRef]
- Santana, I.; Pepe, A.; Jimenez-Pique, E.; Pellice, S.; Milošev, I.; Ceré, S. Corrosion protection of carbon steel by silica-based hybrid coatings containing cerium salts: Effect of silica nanoparticle content. Surf. Coat. Technol. 2015, 265, 106–116. [Google Scholar] [CrossRef]
- Trabelsi, W.; Cecilio, P.; Ferreira, M.G.S.; Montemor, M.F. Electrochemical assessment of the self-healing properties of Ce-doped silane solutions for the pre-treatment of galvanised steel substrates. Prog. Org. Coat. 2005, 54, 276–284. [Google Scholar] [CrossRef]
- Juan-Díaz, M.J.; Martínez-Ibáñez, M.; Hernández-Escolano, M.; Cabedo, L.; Izquierdo, R.; Suay, J.; Gurruchaga, M.; Goñi, I. Study of the degradation of hybrid sol–gel coatings in aqueous medium. Prog. Org. Coat. 2014, 77, 1799–1806. [Google Scholar] [CrossRef]








| Material/Coating | Ds × 10−7 (m2/s) | P (%) | ks (W/(m·K)) | P (%) |
|---|---|---|---|---|
| TMZ-1 | 2.53 | 0.96 | 0.26 | 0.85 |
| TMZ-2 | 2.44 | 0.85 | 0.25 | 0.78 |
| TMZ-3 | 2.05 | 0.58 | 0.21 | 0.49 |
| TMZ-1 + Ce(NO3)3 | 2.14 | 0.88 | 0.22 | 0.73 |
| TMZ-2 + Ce(NO3)3 | 1.96 | 0.76 | 0.20 | 0.67 |
| TMZ-3 + Ce(NO3)3 | 1.75 | 0.51 | 0.18 | 0.42 |
| Material/Coating | Rp (MΩ·cm2) | Ecorr (V) | jcorr (nA/cm2) |
|---|---|---|---|
| Aluminum | 0.013 ± 0.002 | −0.75 ± 0.02 | 296 ± 40 |
| TMZ-1 | 7.7 ± 1.4 | −0.69 ± 0.03 | 5.4 ± 0.2 |
| TMZ-2 | 11.8 ± 2.1 | −0.68 ± 0.02 | 4.1 ± 0.2 |
| TMZ-3 | 14.1 ± 2.4 | −0.65 ± 0.02 | 2.9 ± 0.1 |
| TMZ-1-Ce | 8.4 ± 1.7 | −0.68 ± 0.02 | 3.0 ± 0.2 |
| TMZ-2-Ce | 18.7 ± 2.5 | −0.70 ± 0.02 | 2.2 ± 0.1 |
| TMZ-3-Ce | 30.4 ± 2.8 | −0.62 ± 0.03 | 1.2 ± 0.1 |
| Immersion time | Qcoat × 109 (Ω−1 cm−2 sn) | ncoat | Rcoat (kΩ cm2) | Qoxide × 109 (Ω−1 cm−2 sn) | noxide | Roxide (MΩ cm2) | Qdl × 106 (Ω−1 cm−2 sn) | ndl | Rct (MΩ cm2) |
|---|---|---|---|---|---|---|---|---|---|
| TMZ-3 | |||||||||
| 1 day | 0.93 | 0.974 | 583.1 | 3.84 | 0.642 | 5.02 | 5.08 | 0.894 | 2.12 |
| 2 days | 0.86 | 0.982 | 314.3 | 4.02 | 0.659 | 4.03 | 4.16 | 0.830 | 2.24 |
| 3 days | 0.93 | 0.974 | 209.1 | 4.32 | 0.652 | 3.22 | 5.06 | 0.821 | 2.11 |
| 7 days | 0.92 | 0.977 | 129.2 | 5.04 | 0.682 | 1.58 | 6.22 | 0.790 | 2.10 |
| TMZ-3-Ce | |||||||||
| 1 day | 0.84 | 0.997 | 223.2 | 4.74 | 0.621 | 7.42 | 6.82 | 0.825 | 2.08 |
| 2 days | 0.85 | 0.996 | 85.3 | 5.61 | 0.609 | 5.53 | 11.30 | 0.830 | 2.07 |
| 3 days | 0.87 | 0.996 | 35.3 | 6.05 | 0.611 | 4.53 | 12.97 | 0.818 | 2.10 |
| 4 days | 0.87 | 0.997 | 82.0 | 5.02 | 0.636 | 5.32 | 12.23 | 0.836 | 2.08 |
| 7 days | 0.88 | 0.997 | 25.3 | 7.16 | 0.624 | 3.00 | 13.78 | 0.875 | 1.69 |
| 10 days | 0.90 | 0.997 | 21.2 | 8.58 | 0.626 | 2.04 | 11.59 | 0.851 | 1.51 |
| 17 days | 0.96 | 0.996 | 18.6 | 11.49 | 0.623 | 1.00 | 8.02 | 0.800 | 1.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodič, P.; Katić, J.; Korte, D.; Desimone, P.M.; Franko, M.; Ceré, S.M.; Metikoš-Huković, M.; Milošev, I. The Effect of Cerium Ions on the Structure, Porosity and Electrochemical Properties of Si/Zr-Based Hybrid Sol-Gel Coatings Deposited on Aluminum. Metals 2018, 8, 248. https://doi.org/10.3390/met8040248
Rodič P, Katić J, Korte D, Desimone PM, Franko M, Ceré SM, Metikoš-Huković M, Milošev I. The Effect of Cerium Ions on the Structure, Porosity and Electrochemical Properties of Si/Zr-Based Hybrid Sol-Gel Coatings Deposited on Aluminum. Metals. 2018; 8(4):248. https://doi.org/10.3390/met8040248
Chicago/Turabian StyleRodič, Peter, Jozefina Katić, Dorota Korte, Paula M. Desimone, Mladen Franko, Silvia M. Ceré, Mirjana Metikoš-Huković, and Ingrid Milošev. 2018. "The Effect of Cerium Ions on the Structure, Porosity and Electrochemical Properties of Si/Zr-Based Hybrid Sol-Gel Coatings Deposited on Aluminum" Metals 8, no. 4: 248. https://doi.org/10.3390/met8040248
APA StyleRodič, P., Katić, J., Korte, D., Desimone, P. M., Franko, M., Ceré, S. M., Metikoš-Huković, M., & Milošev, I. (2018). The Effect of Cerium Ions on the Structure, Porosity and Electrochemical Properties of Si/Zr-Based Hybrid Sol-Gel Coatings Deposited on Aluminum. Metals, 8(4), 248. https://doi.org/10.3390/met8040248