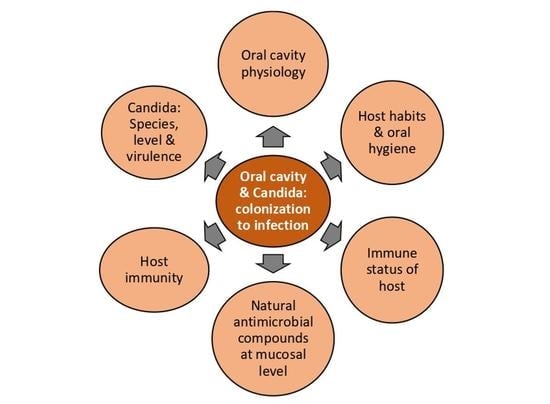
Oral Cavity and Candida albicans: Colonisation to the Development of Infection
Abstract
:1. Introduction
2. Role of Saliva and Mucosa
3. Host Factors and Habits
3.1. Smoking
3.2. Dental Caries
3.3. Oral Prostheses
3.4. Cancer Treatment
3.5. HIV
3.6. Diabetes
3.7. Organ Transplant
4. Candida albicans
4.1. Pathogenicity of Candida albicans
4.2. From Colonszation to Infection
5. Prevention and Treatment
6. Conclusions
7. Future Research
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, P.; Martin, M.V. Oral Microbiology, 4th ed.; Wright Edinburgh: London, UK; New York, NY, USA; Oxford, UK; Philadelphia, PA, USA; St Louis, MO, USA; Sydney, Australia; Toronto, ON, Canada, 2003. [Google Scholar]
- Manning, D.J.; Coughlin, R.P.; Poskit, E.M. Candida in mouth or on dummy? Arch. Dis. Child. 1985, 60, 381–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdicevsky, I.; Ben-Aryeh, H.; Sazargel, R.; Gutman, D. Oral Candida in children. Oral Surg. Oral Med. Oral Pathol. 1984, 57, 37–40. [Google Scholar] [CrossRef]
- Lucas, V.S. Association of psychotropic drugs, prevalence of denture-related stomatitis and oral candidosis. Community Dent. Oral Epidemiol. 1993, 21, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Arendorf, T.M.; Walker, D.M. The prevalence and intra-oral distribution of Candida albicans in man. Arch. Oral Biol. 1980, 25, 1–10. [Google Scholar] [CrossRef]
- Sato, T.; Kishi, M.; Suda, M.; Sakata, K.; Shimoda, H.; Miura, H.; Ogawa, A.; Kobayashi, S. Prevalence of Candida Albicans Non-Albicans the tongue dorsa of elderly people living in a post-disaster area: A cross-sectional survey. BMC Oral Health 2017, 17, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaremba, M.L.; Daniluk, T.; Rozkiewicz, D.; Cylwik-Rokicka, D.; Kierklo, A.; Tokajuk, G.; Dabrowska, E.; Pawińska, M.; Klimiuk, A.; Stokowska, W.; et al. Incidence rate of Candida species in the oral cavity of middle-aged and elderly subjects. Adv. Med. Sci. 2006, 51 (Suppl. 1), 233–236. [Google Scholar] [PubMed]
- Fraser, V.J.; Jones, M.; Dunkel, J.; Storfer, S.; Medoff, G.; Dunagan, W.C. Candidemia in a tertiary care hospital: Epidemiology, risk factors, and predictors of mortality. Clin. Infect. Dis. 1992, 15, 414–421. [Google Scholar] [CrossRef]
- Torres, S.R.; Peixoto, C.B.; Caldas, D.M.; Silva, E.B.; Akiti, T.; Nucci, M.; de Uzeda, M. Relationship between salivary flow rates and Candida counts in subjects with xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 93, 149–154. [Google Scholar] [CrossRef]
- Almståhl, A.; Finizia, C.; Carlén, A.; Fagerberg-Mohlin, B.; Alstad, T. Mucosal microflora in head and neck cancer patients. Int. J. Dent. Hyg. 2018, 16, 459–466. [Google Scholar] [CrossRef]
- Karbach, J.; Walter, C.; Al-Nawas, B. Evaluation of saliva flow rates, Candida colonization and susceptibility of Candida strains after head and neck radiation. Clin. Oral Investig. 2012, 16, 1305–1312. [Google Scholar] [CrossRef]
- Tarapan, S.; Matangkasombut, O.; Trachootham, D.; Sattabanasuk, V.; Talungchit, S.; Paemuang, W.; Phonyiam, T.; Chokchaitam, O.; Mungkung, O.O.; Lam-Ubol, A. Oral Candida colonization in xerostomic postradiotherapy head and neck cancer patients. Oral Dis. 2019, 25, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.R.; Peixoto, C.B.; Caldas, D.M.; Silva, E.B.; Magalhães, F.A.C.; Uzeda, M.; Nucci, M. Clinical aspects of Candida species carriage in saliva of xerotomic subjects. Med. Mycol. 2003, 41, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buranarom, N.; Komin, O.; Matangkasombut, O. Hyposalivation, Oral health, and Candida colonization in independent dentate elders. PLoS ONE 2020, 15, e0242832. [Google Scholar] [CrossRef]
- Nikou, S.A.; Kichik, N.; Brown, R.; Ponde, N.O.; Ho, J.; Naglik, J.R.; Richardson, J.P. Candidaalbicans interactions with mucosal surfaces during health and disease. Pathogens 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontology 2000 2016, 70, 80–92. [Google Scholar] [CrossRef]
- Valentijn-Benz, M.; Nazmi, K.; Brand, H.S.; Hof, W.V.; Veerman, E.C.I. Growth of Candida albicans in human saliva is supported by low-molecular-mass compounds. FEMS Yeast Res. 2015, 15, fov088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abiko, Y.; Saitoh, M.; Nishimura, M.; Yamazaki, M.; Sawamura, D.; Kaku, T. Role of beta-defensins in oral epithelial health and disease. Med. Mol. Morphol. 2007, 40, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Leito, J.T.D.; Ligtenberg, A.J.M.; Nazmi, K.; Veerman, E.C.I. Identification of salivary components that induce transition of hyphae to yeast in Candida albicans. FEMS Yeast Res. 2009, 9, 1102–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar] [CrossRef]
- Xu, T.; Levitz, S.M.; Diamond, R.D.; Oppenheim, F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991, 59, 2549–2554. [Google Scholar] [CrossRef] [Green Version]
- Jainkittivong, A.; Johnson, D.A.; Yeh, C.K. The relationship between salivary histatin levels and oral yeast carriage. Oral Microbiol. Immunol. 1998, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, J.; Kanehira, T.; Mizugai, H.; Chiba, I.; Morita, M. Relationship between salivary histatin 5 levels and Candida CFU counts in healthy elderly. Gerodontology 2006, 23, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.L.; Johnson, D.A.; Patterson, T.F.; Wu, Y.; Lu, D.L.; Shi, Q.; Yeh, C.-K. Salivary anticandidal activity and saliva composition in an HIV-infected cohort. Oral Microbiol. Immunol. 2001, 16, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Ryan, L. Beta-defensins: What are they really doing in the oral cavity? Oral Dis. 2011, 17, 628–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polesello, V.; Segat, L.; Crovella, S.; Zupin, L. Candida infections and human defensins. Protein Pept. Lett. 2017, 24, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Jiang, B.; Chandra, J.; Ghannoum, M.; Nelson, S.; Weinberg, A. Human beta-defensins: Differential activity against candidal species and regulation by Candida albicans. J. Dent. Res. 2005, 84, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Li, X.S.; Berner, J.C.; Edgerton, M. Distinct antifungal mechanisms: Beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob. Agents Chemother. 2006, 50, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, P.W.; Yang, C.Y.; Chang, H.T.; Lan, C.Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 2011, 6, e17755. [Google Scholar] [CrossRef] [Green Version]
- Tomalka, J.; Azodi, E.; Narra, H.P.; Patel, K.; O’Neill, S.; Cardwell, C.; Hall, B.A.; Wilson, J.M.; Hise, A.G. β-Defensin 1 plays a role in acute mucosal defense against Candida albicans. J. Immunol. 2015, 194, 1788–1795. [Google Scholar] [CrossRef] [Green Version]
- Hebecker, B.; Naglik, J.R.; Hube, B.; Jacobsen, I.D. Pathogenicity mechanisms and host response during oral Candida albicans infections. Expert Rev. Anti-Infect. Ther. 2014, 12, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.P.; Moyes, D.L.; Ho, J.; Naglik, J.R. Candida innate immunity at the mucosa. Semin. Cell Dev. Biol. 2019, 89, 58–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertel, M.; Schmidt-Westhausen, A.M.; Strietzel, F.P. Local, systemic, demographic, and health-related factors influencing pathogenic yeast spectrum and antifungal drug administration frequency in oral candidiasis: A retrospective study. Clin. Oral Investig. 2016, 20, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Khammissa, R.A.G.; Chandran, R.; Altini, M.; Lemmer, J. Oral candidosis in relation to oral immunity. J. Oral Pathol. Med. 2014, 43, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimaki, F.; Yamada, S.-I.; Kawamoto, M.; Sakurai, A.; Hayashi, K.; Kurita, H. Relationship between the quantity of oral Candida and systemic condition/diseases of the host: Oral Candida increases with advancing age and anemia. Mycopathologia 2019, 184, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Ellepola, A.N.B. The impact of cigarette/tobacco smoking on oral candidosis: An overview. Oral Dis. 2005, 11, 268–273. [Google Scholar] [CrossRef]
- Darwazeh, A.M.-G.; Al-Dwairi, Z.N.; Al-Zwairi, A.A.-W. The relationship between tobacco smoking and oral colonization with Candida species. J. Contemp. Dent. Pract. 2010, 11, 017–024. [Google Scholar]
- Muzurović, S.; Hukić, M.; Babajić, E.; Smajić, R. The relationship between cigarette smoking and oral colonization with Candida species in healthy adult subjects. Med. Glas. 2013, 10, 397–399. [Google Scholar]
- Sheth, C.C.; Makda, K.; Dilmahomed, Z.; González, R.; Luzi, A.; Del, M.; Jovani-Sancho, M.; Veses, V. Alcohol and tobacco consumption affect the oral carriage of Candida albicans and mutans streptococci. Lett. Appl. Microbiol. 2016, 63, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Mun, M.; Yap, T.; Alnuaimi, A.D.; Adams, G.G.; McCullough, M.J. Oral candidal carriage in asymptomatic patients. Aust. Dent. J. 2016, 61, 190–195. [Google Scholar] [CrossRef]
- Akram, Z.; Al-Kheraif, A.A.; Kellesarian, S.V.; Vohra, F.; Javed, F. Comparison of oral Candida carriage in waterpipe smokers, cigarette smokers, and non-smokers. J. Oral Sci. 2018, 60, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Alrouji, M.; Manouchehrinia, A.; Gran, B.; Constantinescu, C.S. Effects of cigarette smoke on immunity, neuroinflammation and multiple sclerosis. J. Neuroimmunol. 2019, 329, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.P.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J.; et al. Cigarette smoking and variations in systemic immune and inflammation markers. J. Natl. Cancer Inst. 2014, 106, dju294. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P. Oral candidosis: An old disease in new guises. Dent. Update 1990, 17, 36–38. [Google Scholar] [PubMed]
- Macgregor, I.D. Effects of smoking on oral ecology. A review of the literature. Clin. Prev. Dent. 1989, 11, 3–7. [Google Scholar]
- Hise, A.G.; Tomalka, J.; Ganesan, S.; Patel, K.; Hall, B.A.; Brown, G.D.; Fitzgerald, K.A. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 2009, 5, 487–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, P.; Wang, X.; Ge, S.; Chen, W.; Wang, W.; Han, X. Long-term cigarette smoking suppresses NLRP3 inflammasome activation in oral mucosal epithelium and attenuates host defense against Candida albicans in a rat model. Biomed. Pharmacother. 2019, 113, 108597. [Google Scholar] [CrossRef] [PubMed]
- Gunasegar, S.; Himratul-Aznita, W.H. Nicotine enhances the thickness of biofilm and adherence of Candida albicans ATCC 14053 and Candida parapsilosis ATCC 22019. FEMS Yeast Res. 2019, 19, foy123. [Google Scholar] [CrossRef] [PubMed]
- De-la-Torre, J.; Marichalar-Mendia, X.; Varona-Barquin, A.; Marcos-Arias, C.; Eraso, E.; Aguirre-Urizar, J.M.; Quindós, G. Caries and Candida colonisation in adult patients in Basque Country (Spain). Mycoses 2016, 59, 234–240. [Google Scholar] [CrossRef]
- de Carvalho, F.G.; Silva, D.S.; Hebling, J.; Spolidorio, L.C.; Spolidorio, D.M.P. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch. Oral Biol. 2006, 51, 1024–1028. [Google Scholar] [CrossRef]
- Fragkou, S.; Balasouli, C.; Tsuzukibashi, O.; Argyropoulou, A.; Menexes, G.; Kotsanos, N.; Kalfas, S. Streptococcus mutans, Streptococcus sobrinus and Candida albicans in oral samples from caries-free and caries-active children. Eur. Arch. Paediatr. Dent. 2016, 17, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.N.; Al-Maswary, E.A.; Al-Hammadi, Z.O.; Ghoname, N. Salivary Candida species carriage patterns and their relation to caries experience among yemeni children. Oral Health Prev. Dent. 2015, 13, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Moon, Y.; Li, L.; Rustchenko, E.; Wakabayashi, H.; Zhao, X.; Feng, C.; Gill, S.R.; McLaren, S.; Malmstrom, H.; et al. Candida albicans carriage in children with severe early childhood caries (S-ECC) and maternal. PLoS ONE 2016, 11, e0164242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samaranayake, L.P.; Geddes, D.A.; Weetman, D.A.; MacFarlane, T.W. Growth and acid production of Candida albicans in carbohydrate supplemented media. Microbios 1983, 37, 105e115. [Google Scholar]
- Charone, S.; Portela, M.B.; das Chagas, M.S.; de Araújo Soares, R.M.; de AraújoCastro, G.F. Biofilm of Candida albicans from oral cavity of an HIV-infected child: Challenge on enamel microhardness. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Niikawa, H.; Yamashiro, H.; Makihira, S.; Nishimura, M.; Egusa, H.; Furukawa, M.; Setijanto, D.; Hamada, T. In vitro cariogenic potential of Candida albicans. Mycoses 2003, 46, e471–e478. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Scoffield, J.; Wu, R.; Deivanayagam, C.; Zou, J.; Wu, H. Antigen I/II mediates interactions between Streptococcus mutans and Candida albicans. Mol. Oral Microbiol. 2018, 33, 283–291. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef]
- Ellepola, K.; Truong, T.; Liu, Y.; Lin, Q.; Lim, T.K.; Lee, Y.M.; Cao, T.; Koo, H.; Seneviratne, C.J. Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans–Candida albicans mixed-species biofilms. Infect. Immun. 2019, 87, e00339-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zomorodian, K.; Haghighi, N.N.; Rajaee, N.; Pakshir, K.; Tarazooie, B.; Vojdani, M.; Sedaghat, F.; Vosoghi, M. Assessment of Candida species colonization and denture-related stomatitis in complete denture wearers. Med. Mycol. 2011, 49, 208–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghani, F.; Chughtai, M.A.; Shah, S.A. Biochemically assessed pathological activity of oral Candida in denture and non-denture wearers. J. Postgrad. Med. Inst. 2011, 25, 188–198. [Google Scholar]
- Loster, B.W.; Loster, J.; Wieczorek, A.; Ryniewicz, W. Mycological analysis of the oral cavity of patients using acrylic removable dentures. Gastroenterol. Res. Pract. 2012, 2012, 951572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopde, N.; Jawale, B.; Pharande, A.; Chaudhari, L.; Hiremath, V.; Redasani, R. Microbial colonization and their relation with potential cofactors in patients with denture stomatitis. J. Contemp. Dent. Pract. 2012, 13, 456–459. [Google Scholar] [PubMed]
- Gusmão, J.M.R.; Ferreira dos Santos, S.S.; Neisser, M.P.; Jorge, A.O.C.; Faria, M.V. Correlation between factors associated with the removable partial dentures use and Candida spp. in saliva. Gerodontology 2011, 28, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Mothibe, J.V.; Patel, M. Pathogenic characteristics of Candida albicans isolated from oral cavities of denture wearers and cancer patients wearing oral prostheses. Microb. Pathog. 2017, 110, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.P.F.M.; Consani, R.L.X.; Sardi, J.C.O.; Mesquita, M.F.; Silva, M.C.V.S.; Sinhoreti, M.A.C. Biofilm formation in denture base acrylic resins and disinfection method using microwave. J. Res. Pract. Dent. 2014, 2014, 112424. [Google Scholar] [CrossRef] [Green Version]
- Hahnel, S.; Rosentritt, M.; Burgers, R.; Handel, G.; Lang, R. Candida albicans biofilm formation on soft denture liners and efficacy of cleaning protocols. Gerodontology 2012, 29, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Figueiral, M.H.; Sousa-Rodrigues, P.; Fernandes, M.H.; Scully, C. The effect of denture adhesives on Candida albicans growth in vitro. Gerodontology 2012, 29, 348–356. [Google Scholar] [CrossRef]
- Epstein, J.B.; Freilich, M.M.; Le, N.D. Risk factors for oropharyngeal candidiasis in patients who receive radiation therapy for malignant conditions of the head and neck. Oral Surg. Oral Med. Oral Pathol. 1993, 76, 169–174. [Google Scholar] [CrossRef]
- Ramla, S.; Sharma, V.; Patel, M. Influence of cancer treatment on the Candida albicans isolated from the oral cavities of cancer patients. Supportive Care Cancer 2016, 24, 2429–2436. [Google Scholar] [CrossRef]
- de Freitas, E.M.; Nobre, S.A.M.; de Oliveira Pires, M.B.; Faria, R.V.J.; Batista, A.U.D.; Bonan, P.R.F. Oral Candida species in head and neck cancer patients treated by radiotherapy. Auris Nasus Larynx 2013, 40, 400–404. [Google Scholar] [CrossRef]
- Patton, L.L.; Phelan, J.A.; Ramos-Gomez, F.J.; Nittayananta, W.; Shiboski, C.H.; Mbuguye, T.L. Prevalence and classification of HIV-associated oral lesions. Oral Dis. 2002, 8 (Suppl. 2), 98–109. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Shackleton, J.T.; Coogan, M.M. Effect of antifungal treatment on the prevalence of yeasts in HIV-infected subjects. J. Med. Microbiol. 2006, 55, 1279–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owotade, F.J.; Patel, M.; Ralephenya, T.R.M.D.; Vergotine, G. Oral Candida colonization in HIV-positive women: Associated factors and changes following antiretroviral therapy. J. Med. Microbiol. 2013, 62, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Teanpaisan, R.; Nittayananta, W. Prevalence of Candida species in AIDS patients and HIV-free subjects in Thailand. J. Oral Pathol. Med. 1998, 27, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, H.C.; Becker, K.; Fegeler, W.; Basu, S.; Chattopadhya, D.; Baveja, U.; Satyanarayana, S.; Kalghatgi, T.; Murlidhar, A. Oropharyngeal carriage of Candida species in HIV-infected patients in India. Mycoses 2003, 46, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Thanyasrisung, P.; Kesakomol, P.; Pipattanagovit, P.; Youngnak-Piboonratanakit, P.; Pitiphat, W.; Matangkasombut, O. Oral Candida carriage and immune status in Thai human immunodeficiency virus-infected individuals. J. Med. Microbiol. 2014, 63, 753–759. [Google Scholar] [CrossRef]
- Du, X.; Xiong, H.; Yang, Y.; Yan, J.; Zhu, S.; Chen, F. Dynamic study of oral Candida infection and immune status in HIV infected patients during HAART. Arch. Oral Biol. 2020, 115, 104741. [Google Scholar] [CrossRef]
- Challacombe, S.J.; Sweet, S.P. Salivary and mucosal immune responses to HIV and its co-pathogens. Oral Dis. 1997, 3 (Suppl. 1), S79–S84. [Google Scholar] [CrossRef]
- Gordon, S.N.; Cervasi, B.; Odorizzi, P.; Silverman, R.; Aberra, F.; Ginsberg, G.; Estes, J.D.; Paiardini, M.; Frank, I.; Silvestri, G. Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J. Immunol. 2010, 185, 5169–5179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goupil, M.; Cousineau-Côté, V.; Aumont, F.; Sénéchal, S.; Gaboury, L.; Hanna, Z.; Jolicoeur, P.; de Repentigny, L. Defective IL-17- and IL-22-dependent mucosal host response to Candida albicans determines susceptibility to oral candidiasis in mice expressing the HIV-1 transgene. BMC Immunol. 2014, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, D.; Marquis, M.; Aumont, F.; Lussier-Morin, A.-C.; Raymond, M.; Sénéchal, S.; Hanna, Z.; Jolicoeur, P.; de Repentigny, L. Altered CD4+ T cell phenotype and function determine the susceptibility to mucosal candidiasis in transgenic mice expressing HIV-1. J. Immunol. 2006, 77, 479–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soysa, N.S.; Samaranayake, L.P.; Ellepola, A.N. Diabetes mellitus as a contributory factor in oral candidosis. Diabet. Med. 2006, 23, 455–459. [Google Scholar] [CrossRef]
- Zomorodian, K.; Kavoosi, F.; Pishdad, G.R.; Mehriar, P.; Ebrahimi, H.; Bandegani, A.; Pakshirb, K. Prevalence of oral Candida colonization in patients with diabetes mellitus. J. Mycol. Médicale 2016, 26, 103–110. [Google Scholar] [CrossRef]
- Matic Petrovic, S.; Radunovic, M.; Barac, M.; Kuzmanovic Pficer, J.; Pavlica, D.; Arsic Arsenijevic, V.; Pucar, A. Subgingival areas as potential reservoirs of different Candida spp. in type 2 diabetes patients and healthy subjects. PLoS ONE 2019, 14, e0210527. [Google Scholar] [CrossRef] [Green Version]
- Contaldo, M.; Romano, A.; Mascitti, M.; Fiori, F.; Della Vella, F.; Serpico, R.; Santarelli, A. Association between denture stomatitis, Candida species and diabetic status. J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. 1), 35–41. [Google Scholar] [PubMed]
- Mishra, N.; Trivedi, A.; Gajdhar, S.K.; Bhagwat, H.; Khutwad, G.K.; Mall, P.E.; Kulkarni, D. Correlation of blood glucose levels, salivary glucose levels and oral colony forming units of Candida albicans in type 2 diabetes mellitus patients. J. Contemp. Dent. Pract. 2019, 20, 494–498. [Google Scholar] [PubMed]
- Javed, F.; Al-Kheraif, A.A.; Kellesarian, S.V.; Vohra, F.; Romanos, G.E. Oral Candida carriage and species prevalence in denture stomatitis patients with and without diabetes. J. Biol. Regul. Homeost. Agents 2017, 31, 343–346. [Google Scholar] [PubMed]
- Patterson, J.E. Epidemiology of fungal infections in solid organ transplant patients. Transpl. Infect. Dis. 1999, 1, 229–236. [Google Scholar] [CrossRef]
- Badiee, P.; Kordbacheh, P.; Alborzi, A.; Zeini, F.; Mirhendy, H.; Mahmoody, M. Fungal infections in solid organ recipients. Exp. Clin. Transplant. 2005, 3, 385–389. [Google Scholar]
- Gupta, K.L.; Ghosh, A.K.; Kochhar, R.; Jha, V.; Chakrabarti, A.; Sakhuja, V. Esophageal candidiasis after renal transplantation: Comparative study in patients on different immunosuppressive protocols. Am. J. Gastroenterol. 1994, 89, 1062–1065. [Google Scholar] [PubMed]
- Patel, R.; Portela, D.; Badley, A.D.; Harmsen, W.S.; Larson-Keller, J.J.; Ilstrup, D.M.; Keating, M.R.; Wiesner, R.H.; Krom, R.A.; Paya, C.V. Risk factor for invasive Candida and non-Candida fungal infections after liver transplantation. Transplantation 1996, 62, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Siahi-Benlarbi, R.; Nies, S.M.; Sziegoleit, A.; Bauer, J.; Schranz, D.; Wetzel, W.E. Caries-, Candida- and Candida antigen/antibody frequency in children after heart transplantation and children with congenital heart disease. Pediatr. Transpl. 2010, 14, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Chaves, G.M.; Diniz, M.G.; da Silva-Rocha, W.P.; de Souza, L.B.; Gondim, L.A.; Ferreira, M.A.; Svidzinski, T.I.; Milan, E.P. Species distribution and virulence factors of Candida spp. isolated from the oral cavity of kidney transplant recipients in Brazil. Mycopathologia 2013, 175, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Dongari-Bagtzoglou, A.; Dwivedi, P.; Ioannidou, E.; Shaqman, M.; Hull, D.; Burleson, J. Oral Candida infection and colonization in solid organ transplant recipients. Oral Microbiol. Immunol. 2009, 24, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; He, C.; Zhao, C.; Chen, X.; Hua, H.; Yan, Z. Characterization of oral candidiasis and the Candida species profile in patients with oral mucosal diseases. Microb. Pathog. 2019, 134, 103575. [Google Scholar] [CrossRef] [PubMed]
- Redding, S.W.; Dahiya, C.; Kirkpatrick, W.R.; Coco, B.J.; Patterson, T.F.; Fothergill, A.W.; Rinaldi, M.G.; Thomas, C.R., Jr. Candida glabrata is an emerging cause of oropharyngeal candidiasis in patients receiving radiation for head and neck cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radio. Endod. 2004, 97, 47–52. [Google Scholar] [CrossRef]
- Naglik, J.R.; König, A.; Hube, B.; Gaffen, S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.N.; Solis, N.V.; Phan, Q.T.; Bajwa, J.S.; Kashleva, H.; Thompson, A.; Liu, Y.; Dongari-Bagtzoglou, A.; Edgerton, M.; Filler, S.G. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010, 6, e1001181. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Phan, Q.T.; Boontheung, P.; Solis, N.V.; Loo, J.A.; Filler, S.G. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc. Natl. Acad. Sci. USA 2012, 109, 14194–14199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, C.A.; Bendel, C.M.; McClellan, M.; Hauser, M.; Becker, J.M.; Berman, J.; Hostetter, M.K. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 1998, 279, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Fostira, F.; Ruprai, J.; Staab, J.F.; Challacombe, S.J.; Sundstrom, P.J. Candida albicans HWP1 gene expression and host antibody responses in colonization and disease. J. Med. Microbiol. 2006, 55 Pt 10, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, P.; Balish, E.; Allen, C.M. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J. Infect. Dis. 2002, 185, 521–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorgo, A.G.; Heilmann, C.J.; Brul, S.; de Koster, C.G.; Kli, F.M. Beyond the wall: Candida albicans secret(e)s to survive. FEMS Microbiol. Lett. 2013, 338, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.S.; Mirbod, F.; Filler, S.G.; Banno, Y.; Cole, G.T.; Kitajima, Y.; Edwards Jr, J.E.; Nozawa, Y.; Ghannoum, M.A. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect. Immun. 1995, 63, 1993–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, J.W.; Bell, A.C.; Hube, B.; Schaller, M.; Warner, T.F.; Balish, E. Candida albicans PLD I activity is required for full virulence. Med. Mycol. 2004, 42, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Seshan, K.R.; Leidich, S.D.; Chandra, J.; Cole, G.T.; Ghannoum, M.A. Reintroduction of the PLB1 gene into Candida albicans restores virulence in vivo. Microbiology 2001, 147 Pt 9, 2585–2597. [Google Scholar] [CrossRef] [Green Version]
- Schofield, D.A.; Westwater, C.; Warner, T.; Balish, E. Differential Candida albicans lipase gene expression during alimentary tract colonization and infection. FEMS Microbiol. Lett. 2005, 244, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Runglall, M.; Murciano, C.; Shen, C.; Nayar, D.; Thavaraj, S.; Kohli, A.; Islam, A.; Mora-Montes, H.; Challacombe, S.J.; et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010, 8, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyes, D.L.; Richardson, J.P.; Naglik, J.R. Candida albicans-epithelial interactions and pathogenicity mechanisms: Scratching the surface. Virulence 2015, 6, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.B.; Pearsall, N.N.; Truelove, E.L. Quantitative relationships between Candida albicans in saliva and the clinical status of human subjects. J. Clin. Microbiol. 1980, 12, 475–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.Y.; Hu, B.Q. Research of oral candidiasis diagnosis. Zhonghua Kou Qiang Yi Xue Za Zhi 1993, 28, 368–371. [Google Scholar] [PubMed]
- Coleman, D.C.; Bennett, D.E.; Gallagher, P.J. Oral candidiasis and HIV infection: Antifungal drug resistance and changes in Candida population dynamics. In Oral Manifestations of HIV Infection; Greenspan, J.S., Greenspan, D., Eds.; Quintessence Publishing Co, Inc.: Chicago, IL, USA, 1995; pp. 112–118. [Google Scholar]
- Tooyama, H.; Matsumoto, T.; Hayashi, K.; Kurashina, K.; Kurita, H.; Uchida, M.; Kasuga, E.; Honda, T. Candida concentrations determined following concentrated oral rinse culture reflect clinical oral signs. BMC Oral Health 2015, 15, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.R.; Hua, H.; Liu, X.S. Quantity of Candida colonies in saliva: A diagnostic evaluation for oral candidiasis. Chin. J. Dent. Res. 2017, 20, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sweet, S.P.; Cookson, S.; Challacombe, S.J. Candida albicans isolates from HIV-infected and AIDS patients exhibit enhanced adherence to epithelial cells. J. Med. Microbiol. 1995, 43, 452–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owotade, F.J.; Patel, M. Virulence of oral Candida isolated from HIV-positive women with oral candidiasis and asymptomatic carriers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 455–460. [Google Scholar] [CrossRef]
- Patel, P.N.; Sah, P.; Chandrashekar, C.; Vidyasagar, S.; Rao, J.V.; Tiwari, M.; Radhakrishnan, R. Oral candidal speciation, virulence and antifungal susceptibility in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2017, 125, 10–19. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.; Barros, L.; Silva, S.; Henriques, M. Candidiasis: Predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 2014, 177, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Shackleton, J.A.; Coogan, M.M.; Galpin, J. Antifungal effect of mouth rinses on oral Candida counts and salivary flow in treatment-naïve HIV-infected patients. AIDS Patient Care STDS 2008, 22, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Pericolini, E.; Paulone, S.; Orsi, C.F.; Castagnoli, A.; Oliva, I.; Strozzi, E.; Blasi, E. In vitro effects of commercial mouthwashes on several virulence traits of Candida albicans, viridans streptococci and Enterococcus faecalis colonizing the oral cavity. PLoS ONE 2018, 13, e0207262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, F.C.; Rossoni, R.D.; de Barros, P.P.; Santos, J.D.; Fugisaki, L.R.O.; Leão, M.P.V.; Junqueira, J.C. Action mechanisms of probiotics on Candida spp. and candidiasis prevention: An update. J. Appl. Microbiol. 2020, 129, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trindade, L.A.; de Araújo Oliveira, J.; Dias de Castro, R.; de Oliveira Lima, E. Inhibition of adherence of C. albicans to dental implants and cover screws by Cymbopogon nardus essential oil and citronellal. Clin. Oral Investig. 2015, 19, 2223–2231. [Google Scholar] [CrossRef]
- Veilleux, M.-P.; Grenier, D. Determination of the effects of cinnamon bark fractions on Candida albicans and oral epithelial cells. BMC Complement. Altern. Med. 2019, 19, 303. [Google Scholar] [CrossRef] [Green Version]
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.; Rehder, V.L.; Duarte, M.C.; Höfling, J.F. Action of Coriandrum sativum L. Essential oil upon oral Candida albicans biofilm formation. Evid. Based Complement. Altern. Med. 2011, 2011, 985832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naicker, S.D.; Patel, M. Dodonaea viscosa var. angustifolia inhibits germ tube and biofilm formation by C. albicans. Evid. Based Complement. Altern. Med. 2013, 2013, 261978. [Google Scholar] [CrossRef] [Green Version]
- Ngabaza, T.; Moeno, S.; Patel, M. Anti-acidogenic and anti-biofilm activity of 5,6,8-trihydroxy-7-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one. Microb. Pathog. 2018, 123, 149–152. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.S.; El-Ganiny, A.M. Back to Nature: Combating Candida albicans biofilm, phospholipase and hemolysin using plant essential oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef]
- Serra, E.; Hidalgo-Bastida, L.A.; Verran, J.; Williams, D.; Malic, S. Antifungal activity of commercial essential oils and biocides against Candida albicans. Pathogens 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabzghabaee, A.M.; Shirdare, Z.; Ebadian, B.; Aslani, A.; Ghannadi, A. Clinical evaluation of the essential oil of Pelargonium graveolens for the treatment of denture stomatitis. Dent. Res. J. 2011, 8 (Suppl. 1), S105–S108. [Google Scholar]
- Vazquez, J.A.; Zawawi, A.A. Efficacy of alcohol-based and alcohol-free melaleuca oral solution for the treatment of fluconazole-refractory oropharyngeal candidiasis in patients with AIDS. HIV Clin. Trials 2002, 3, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Catalán, A.; Pacheco, J.G.; Martínez, A.; Mondaca, M.A. In vitro and in vivo activity of Melaleuca alternifolia mixed with tissue conditioner on Candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 105, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Jandourek, A.; Vaishampayan, J.K.; Vazquez, J.A. Efficacy of melaleuca oral solution for the treatment of fluconazole refractory oral candidiasis in AIDS patients. AIDS 1998, 12, 1033–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Local factors and habits |
|
| Systemic factors |
|
| Medications |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M. Oral Cavity and Candida albicans: Colonisation to the Development of Infection. Pathogens 2022, 11, 335. https://doi.org/10.3390/pathogens11030335
Patel M. Oral Cavity and Candida albicans: Colonisation to the Development of Infection. Pathogens. 2022; 11(3):335. https://doi.org/10.3390/pathogens11030335
Chicago/Turabian StylePatel, Mrudula. 2022. "Oral Cavity and Candida albicans: Colonisation to the Development of Infection" Pathogens 11, no. 3: 335. https://doi.org/10.3390/pathogens11030335
APA StylePatel, M. (2022). Oral Cavity and Candida albicans: Colonisation to the Development of Infection. Pathogens, 11(3), 335. https://doi.org/10.3390/pathogens11030335