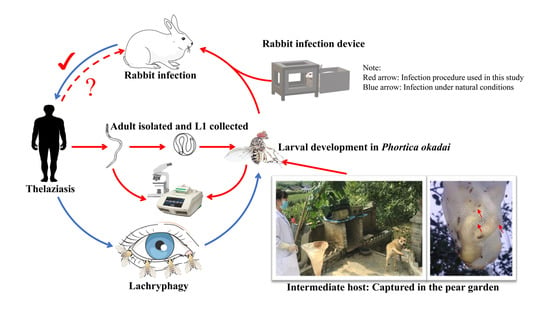
Laboratory Culture and Life Cycle of Thelazia callipaeda in Intermediate and Definitive Hosts
Abstract
:1. Introduction
2. Materials and Methods
2.1. T. callipaeda Collection and Identification
2.2. P. okadai Colonies
2.3. Design and Analysis of P. okadai Infection Procedure
2.4. Design and Analysis of Rabbit Infection Procedure
3. Results
3.1. T. callipaeda Morphological Identification
3.2. Molecular Analysis
3.3. Morphological Characteristics of L1
3.4. Larval Development in P. okadai
3.5. Rabbit Infection Is Achievable
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otranto, D.; Cantacessi, C.; Dantas-Torres, F.; Brianti, E.; Pfeffer, M.; Genchi, C.; Guberti, V.; Capelli, G.; Deplazes, P. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Vet. Parasitol. 2015, 213, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Mendoza-Roldan, J.A.; Dantas-Torres, F. Thelazia callipaeda. Trends Parasitol. 2021, 37, 263–264. [Google Scholar] [CrossRef]
- Wang, Z.X.; Yang, Z.X. Studies on the development of Thelazia callipaeda larvae in the intermediate host Amiota variegata in China. Chin. J. Zool. 1993, 28, 4–8. [Google Scholar]
- Otranto, D.; Lia, R.P.; Buono, V.; Traversa, D.; Giangaspero, A. Biology of Thelazia callipaeda (Spirurida, Thelaziidae) eyeworms in naturally infected definitive hosts. Parasitology 2004, 129, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Testini, G.; De Luca, F.; Hu, M.; Shamsi, S.; Gasser, R.B. Analysis of genetic variability within Thelazia callipaeda (Nematoda: Thelazioidea) from Europe and Asia by sequencing and mutation scanning of the mitochondrial cytochrome coxidase subunit 1 gene. Mol. Cell. Probes 2005, 19, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Iatta, R.; Lia, R.P.; Cavalera, M.A.; Maca, J.; Pombi, M.; Dantas-Torres, F.; Jaenike, J. Phortica variegata Competence of from the United States as an Intermediate Host of the Eyeworm. Am. J. Trop. Med. Hyg. 2018, 98, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.R.; Spoerel, S.; Wiesner, L.; Klein, M.; Pantchev, N.; Taubert, A.; Hermosilla, C. Ophthalmic Thelazia callipaeda infections: First feline and new canine imported cases in Germany. Parasitol. Res. 2020, 119, 3099–3104. [Google Scholar]
- Papadopoulos, E.; Komnenou, A.; Thomas, A.; Ioannidou, E.; Colella, V.; Otranto, D. Spreading of Thelazia callipaeda in Greece. Transbound Emerg. Dis. 2018, 65, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Carroll, F.E. Thelazia infection of man and mammals in China. Trans. Royal Soc. Trop. Med. Hyg. 1927, 20, 365–369. [Google Scholar]
- Shen, J.L.; Gasser, R.B.; Chu, D.Y.; Wang, Z.X.; Yuan, X.S.; Cantacessi, C.; Otranto, D. Human Thelaziosis a Neglected Parasitic Disease of the Eye. J. Parasitol. 2006, 92, 872–875. [Google Scholar] [CrossRef]
- Liu, S.N.; Xu, F.F.; Chen, W.Q.; Jiang, P.; Cui, J.; Wang, Z.Q.; Zhang, X. A Case of Human Thelaziasis and Review of Chinese Cases. Acta Parasitol. 2020, 65, 783–786. [Google Scholar] [CrossRef]
- Huang, J.; Gong, L.U.; Tsaur, S.C.; Zhu, L.; An, K.; Chen, H. Revision of the subgenus Phortica (sensu stricto) (Diptera, Drosophilidae) from East Asia, with assessment of species delimitation using DNA barcodes. Zootaxa 2019, 4678, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Catarino, A.; Almeida, B.; Ramos, C.; Campino, L.; Cardoso, L. Emergence of Thelazia callipaeda Infection in Dogs and Cats from East-Central Portugal. Transbound Emerg. Dis. 2019, 63, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Gasser, R.B.; Otranto, D.; Xu, M.; Shen, J.L.; Mohandas, N.; Zhou, D.H.; Zhu, X.Q. Mitochondrial genome of the eyeworm, Thelazia callipaeda (Nematoda: Spirurida), as the first representative from the family Thelaziidae. PLoS Negl. Trop. Dis. 2013, 7, e2029. [Google Scholar] [CrossRef]
- Cai, J.; Huang, L.; Tang, H.R.; Xu, H.L.; Wang, L.J.; Zheng, M.H.; Yu, H.; Liu, H. Macrophage migration inhibitory factor of Thelazia callipaeda induces M2-like macrophage polarization through TLR4-mediated activation of the PI3K-Akt pathway. FASEB J. 2021, 35, e21866. [Google Scholar] [CrossRef]
- Ren, X.H. Morphological Observation and Genetic Variability Study of Thelazia callipaeda in Zunyi and Surrounding Regions. Master’s Thesis, Zunyi Medical University, Zunyi, China, 2016. [Google Scholar]
- Cabanova, V.; Miterpakova, M.; Oravec, M.; Hurnikova, Z.; Jerg, S.; Nemcikova, G.; Červenská, M.B. Nematode Thelazia callipaeda is spreading across Europe. The first survey of red foxes from Slovakia. Acta Parasitol. 2018, 63, 160–166. [Google Scholar] [CrossRef]
- Marino, V.; Galvez, R.; Colella, V.; Sarquis, J.; Checa, R.; Montoya, A.; Barrera, J.P.; Domínguez, S.; Lia, R.P.; Otranto, D.; et al. Detection of Thelazia callipaeda in Phortica variegata and spread of canine thelaziosis to new areas in Spain. Parasites Vectors 2018, 11, 195. [Google Scholar] [CrossRef]
- Huang, X.G.; Zhang, L.F.; Wang, L.J.; Zheng, M.H.; Liu, H. The zoophilic fruit fly Amiota okadai in Zunyi City: Flies capture, morphology identification and laboratory breeding. Med. Pest Control 2017, 33, 765–770. [Google Scholar]
- Wang, Z.X.; Wang, K.C.; Shen, J.L.; Hu, Y.; Chen, Q.; Wang, H.Y.; Zhang, L.W.; Wang, Z.C.; Jiang, B.L. Capturing and Identification of Amiota okadai, the Intermediate Host of Thelazia callipaeda. Chin. J. Zool. 2002, 3, 58–61. [Google Scholar]
- Otranto, D.; Lia, R.P.; Cantacessi, C.; Testini, G.; Troccoli, A.; Shen, J.L.; Wang, Z.X. Nematode biology and larval development of Thelazia callipaeda (Spirurida, Thelaziidae) in the drosophilid intermediate host in Europe and China. Parasitology 2005, 131, 847–855. [Google Scholar] [CrossRef]
- Rolbiecki, L.; Izdebska, J.N.; Franke, M.; Iliszko, L.; Fryderyk, S. The Vector-Borne Zoonotic Nematode Thelazia callipaeda in the Eastern Part of Europe, with a Clinical Case Report in a Dog in Poland. Pathogens 2021, 10, 55. [Google Scholar] [CrossRef]
- Ionica, A.M.; Deak, G.; D’amico, G.; Stan, G.F.; Chisamera, G.B.; Constantinescu, I.C.; Adam, C.; Lefkaditis, M.; Gherman, C.M.; Mihalca, A.D. Thelazia callipaeda in mustelids from Romania with the European badger, Meles meles, as a new host for this parasite. Parasites Vectors 2019, 12, 370. [Google Scholar] [CrossRef]
- Palfreyman, J.; Graham-Brown, J.; Caminade, C.; Gilmore, P.; Otranto, D.; Williams, D.J.L. Predicting the distribution of Phortica variegata and potential for Thelazia callipaeda transmission in Europe and the United Kingdom. Parasites Vectors 2018, 11, 272. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Y.L.; Han, L.L.; Xiong, C.; Yi, S.Q.; Jiang, P.; Wang, Z.X.; Shen, J.L.; Cui, J.; Wang, Z.Q. Population structure analysis of the neglected parasite Thelazia callipaeda revealed high genetic diversity in Eastern Asia isolates. PLoS Negl. Trop. Dis. 2018, 12, e0006165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Z.X. Experimental observation on development and reproduction of Thelazia callipaeda in definitive host. Chin. J. Zool. 1993, 28, 5–11. [Google Scholar]
- Morgado, A.C.T.; do Vale, B.; Ribeiro, P.; Coutinho, T.; Santos-Silva, S.; de Sousa Moreira, A.; Rodrigues, F.T.; Coelho, A.C.; Lopes, A.P.; Mesquita, J.R.; et al. First report of human Thelazia callipaeda infection in Portugal. Acta Trop. 2022, 231, 106436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Shen, J.L.; Wang, H.Y.; Otranto, D. An Update on the Research of Human Thelaziosis. Chin. J. Parasitol. Parasit. Dis. 2006, 24, 299–303. [Google Scholar]
- Abuseir, S. Meat-borne parasites in the Arab world: A review in a One Health perspective. Parasitol. Res. 2021, 120, 4153–4166. [Google Scholar] [CrossRef]
- Otranto, D.; Strube, C.; Xiao, L.H. Zoonotic parasites: The One Health challenge. Parasitol. Res. 2021, 120, 4073–4074. [Google Scholar] [CrossRef]
- Fooks, A.R.; Johnson, N. Jet set pets: Examining the zoonosis risk in animal import and travel across the European Union. Vet. Med. 2015, 6, 17–25. [Google Scholar]
- Cao, H.; Wang, X.; Gao, J.; Prigent, S.R.; Watabe, H.; Zhang, Y.; Chen, H. Phylogeny of the African and Asian Phortica (Drosophilidae) deduced from nuclear and mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2011, 61, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Lebon, W.; Guillot, J.; Álvarez, M.J.; Antonio Bazaga, J.; Cortes-Dubly, M.L.; Dumont, P.; Eberhardt, M.; Gómez, H.; Pennant, O.; Siméon, N.; et al. Prevention of canine ocular thelaziosis (Thelazia callipaeda) with a combination of milbemycin oxime and afoxolaner (Nexgard Spectra) in endemic areas in France and Spain. Parasite 2019, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Colella, V.; Crescenzo, G.; Solari Basano, F.; Nazzari, R.; Capelli, G.; Petry, G.; Schaper, R.; Pollmeier, M.; Mallia, E.; et al. Efficacy of moxidectin 2.5% and imidacloprid 10% in the treatment of ocular thelaziosis by Thelazia callipaeda in naturally infected dogs. Vet. Parasitol. 2016, 227, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Graham-Brown, J.; Gilmore, P.; Colella, V.; Moss, L.; Dixon, C.; Andrews, M.; Arbeid, P.; Barber, J.; Timofte, D.; McGarry, J.; et al. Three cases of imported eyeworm infection in dogs: A new threat for the United Kingdom. Vet. Rec. 2017, 181, 346. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, D.; Yin, C.; Tang, H.; Luo, B.; Yan, R.; Shen, Y.; Liu, H. Laboratory Culture and Life Cycle of Thelazia callipaeda in Intermediate and Definitive Hosts. Pathogens 2022, 11, 1066. https://doi.org/10.3390/pathogens11091066
Wang L, Li D, Yin C, Tang H, Luo B, Yan R, Shen Y, Liu H. Laboratory Culture and Life Cycle of Thelazia callipaeda in Intermediate and Definitive Hosts. Pathogens. 2022; 11(9):1066. https://doi.org/10.3390/pathogens11091066
Chicago/Turabian StyleWang, Lingjun, Di Li, Changzhu Yin, Hongri Tang, Bo Luo, Rong Yan, Yujuan Shen, and Hui Liu. 2022. "Laboratory Culture and Life Cycle of Thelazia callipaeda in Intermediate and Definitive Hosts" Pathogens 11, no. 9: 1066. https://doi.org/10.3390/pathogens11091066
APA StyleWang, L., Li, D., Yin, C., Tang, H., Luo, B., Yan, R., Shen, Y., & Liu, H. (2022). Laboratory Culture and Life Cycle of Thelazia callipaeda in Intermediate and Definitive Hosts. Pathogens, 11(9), 1066. https://doi.org/10.3390/pathogens11091066