Novel Molecular Consortia of Cannabidiol with Nonsteroidal Anti-Inflammatory Drugs Inhibit Emerging Coronaviruses’ Entry
Abstract
:1. Introduction
- the design of new chemical systems based on innovative structural features [1], such as hybrids and conjugates (molecular consortia in general);
- the use of compounds of natural origin, i.e., phytochemicals, with pleiotropic activity, which have been effective in the treatment of many diseases [2];
- the use of small-molecule drugs and their new derivatives, with known and proven therapeutic power [3].
2. Materials and Methods
2.1. General Procedure for the Synthesis of CBD-NSAID Molecular Consortia
2.2. Cells and Viruses
2.3. The Cytotoxicity Analysis of CBD-NSAID Molecular Consortia
2.4. CBD-NSAID-Mediated Inhibition of SARS-CoVs Virus Entry
2.5. The CBD-NSAID Molecular Consortia’s Effect on Influenza A Virus Replication
2.6. Statistical Analysis
3. Results
3.1. NSAID-CBD Molecular Consortia Synthesis
3.2. The Cytotoxicity of CBD-NSAID Molecular Consortia
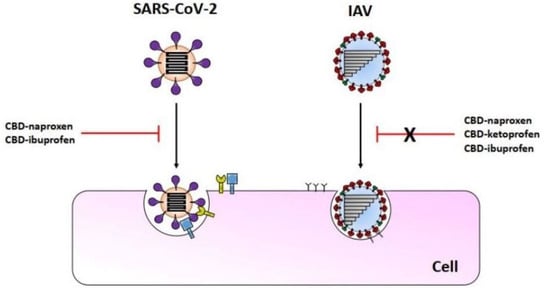
3.3. The CBD-NSAIDs Effect on the SARS-CoVs Entry
3.4. The CBD-NSAIDs Effect on the IAV Replication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawełczyk, A.; Sowa-Kasprzak, K.; Olender, D.; Zaprutko, L. Molecular Consortia—Various Structural and Synthetic Concepts for More Effective Therapeutics Synthesis. Int. J. Mol. Sci. 2018, 19, 1104. [Google Scholar] [CrossRef] [Green Version]
- Mihaylova, D.; Popova, A. Phytochemicals of Natural Products: Analysis and Biological Activities. Horticulturae 2023, 9, 167. [Google Scholar] [CrossRef]
- Lu, B.; Atala, A. Small molecules and small molecule drugs in regenerative medicine. Drug Discov. Today 2014, 19, 801–808. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. [Google Scholar] [CrossRef]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol--recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [Green Version]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Zuardi, A.W.; Crippa, J.A.S. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals 2012, 5, 529–552. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Green, R.; Khalil, R.; Mohapatra, S.S.; Mohapatra, S. Role of Cannabidiol for Improvement of the Quality of Life in Cancer Patients: Potential and Challenges. Int. J. Mol. Sci. 2022, 23, 12956. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salles, É.L.; Khodadadi, H.; Jarrahi, A.; Ahluwalia, M.; Paffaro, V.A.; Costigliola, V.; Yu, J.C.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J. Cell. Mol. Med. 2020, 24, 12869–12872. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Jarrahi, A.; Chibane, F.; Costigliola, V.; Yu, J.C.; Vaibhav, K.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol Modulates Cytokine Storm in Acute Respiratory Distress Syndrome Induced by Simulated Viral Infection Using Synthetic RNA. Cannabis Cannabinoid Res. 2020, 5, 197–201. [Google Scholar] [CrossRef]
- Wang, B.; Kovalchuk, A.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. In search of preventive strategies: Novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging 2020, 12, 22425–22444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Luo, Y.; Zhu, Y.; Chen, Q.; Qiu, J.; Cong, F.; Li, Y.; Zhang, X. Nonsteroidal anti-inflammatory drugs (NSAIDs) and nucleotide analog GS-441524 conjugates with potent in vivo efficacy against coronaviruses. Eur. J. Med. Chem. 2023, 249, 115113. [Google Scholar] [CrossRef]
- Ahmadi, M.; Bekeschus, S.; Weltmann, K.-D.; von Woedtke, T.; Wende, K. Non-steroidal anti-inflammatory drugs: Recent advances in the use of synthetic COX-2 inhibitors. RSC Med. Chem. 2022, 13, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Lolli, M.L.; Cena, C.; Medana, C.; Lazzarato, L.; Morini, G.; Coruzzi, G.; Manarini, S.; Fruttero, R.; Gasco, A. A new class of ibuprofen derivatives with reduced gastrotoxicity. J. Med. Chem. 2001, 44, 3463–3468. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Papierska, K.; Narożna, M.; Jelińska, A.; Majchrzak-Celińska, A. Cannabidiol and Its Combinations with Nonsteroidal Anti-Inflammatory Drugs Induce Apoptosis and Inhibit Activation of NF-κB Signaling in Vulvar Squamous Cell Carcinoma. Molecules 2022, 27, 8779. [Google Scholar] [CrossRef]
- Hanika, A.; Larisch, B.; Steinmann, E.; Schwegmann-Weßels, C.; Herrler, G.; Zimmer, G. Use of influenza C virus glycoprotein HEF for generation of vesicular stomatitis virus pseudotypes. J. Gen. Virol. 2005, 86, 1455–1465. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Gnirß, K.; Zmora, P.; Blazejewska, P.; Winkler, M.; Lins, A.; Nehlmeier, I.; Gärtner, S.; Moldenhauer, A.-S.; Hofmann-Winkler, H.; Wolff, T.; et al. Tetherin Sensitivity of Influenza A Viruses Is Strain Specific: Role of Hemagglutinin and Neuraminidase. J. Virol. 2015, 89, 9178–9188. [Google Scholar] [CrossRef] [Green Version]
- Piasecka, J.; Lenartowicz, E.; Soszynska-Jozwiak, M.; Szutkowska, B.; Kierzek, R.; Kierzek, E. RNA Secondary Structure Motifs of the Influenza A Virus as Targets for siRNA-Mediated RNA Interference. Mol. Ther. Nucleic Acids 2019, 19, 627–642. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Zmora, P.; Wrensch, F.; Herrler, G.; Pöhlmann, S. The Hemagglutinin of Bat-Associated Influenza Viruses Is Activated by TMPRSS2 for pH-Dependent Entry into Bat but Not Human Cells. PLoS ONE 2016, 11, e0152134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawełczyk, A.; Olender, D.; Sowa-Kasprzak, K.; Zaprutko, L. Hybrid Compounds Strategy in the Synthesis of Oleanolic Acid Skeleton-NSAID Derivatives. Molecules 2016, 21, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenvi, S.; Kiran, K.R.; Kumar, K.; Diwakar, L.; Reddy, G.C. Synthesis and biological evaluation of boswellic acid-NSAID hybrid molecules as anti-inflammatory and anti-arthritic agents. Eur. J. Med. Chem. 2015, 98, 170–178. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 24 March 2023).
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet Lond. Engl. 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef]
- EMA COVID-19 Treatments. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments (accessed on 24 March 2023).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef]
- Robinson, P.C.; Liew, D.F.L.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.-P.; Hussell, T.; et al. COVID-19 therapeutics: Challenges and directions for the future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef]
- Robb, C.T.; Goepp, M.; Rossi, A.G.; Yao, C. Non-steroidal anti-inflammatory drugs, prostaglandins, and COVID-19. Br. J. Pharmacol. 2020, 177, 4899–4920. [Google Scholar] [CrossRef]
- Kragholm, K.; Torp-Pedersen, C.; Fosbol, E. Non-steroidal anti-inflammatory drug use in COVID-19. Lancet Rheumatol. 2021, 3, e465–e466. [Google Scholar] [CrossRef]
- Kushner, P.; McCarberg, B.H.; Grange, L.; Kolosov, A.; Haveric, A.L.; Zucal, V.; Petruschke, R.; Bissonnette, S. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in COVID-19. Npj Prim. Care Respir. Med. 2022, 32, 35. [Google Scholar] [CrossRef]
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8, 989. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Gula, H.; Saxena, D.; Gabbard, J.D.; Chen, S.-N.; Ohtsuki, T.; Friesen, J.B.; et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci. Adv. 2022, 8, eabi6110. [Google Scholar] [CrossRef]
- Corpetti, C.; Del Re, A.; Seguella, L.; Palenca, I.; Rurgo, S.; De Conno, B.; Pesce, M.; Sarnelli, G.; Esposito, G. Cannabidiol inhibits SARS-CoV-2 spike (S) protein-induced cytotoxicity and inflammation through a PPARγ-dependent TLR4/NLRP3/Caspase-1 signaling suppression in Caco-2 cell line. Phytother. Res. 2021, 35, 6893–6903. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef]
- Zheng, W.; Fan, W.; Zhang, S.; Jiao, P.; Shang, Y.; Cui, L.; Mahesutihan, M.; Li, J.; Wang, D.; Gao, G.F.; et al. Naproxen Exhibits Broad Anti-influenza Virus Activity in Mice by Impeding Viral Nucleoprotein Nuclear Export. Cell Rep. 2019, 27, 1875–1885.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Structure-Based Design of Novel Naproxen Derivatives Targeting Monomeric Nucleoprotein of Influenza a Virus. Available online: https://www.tandfonline.com/doi/epdf/10.1080/07391102.2014.979230?src=getftr (accessed on 24 March 2023).
- Structure-Based Discovery of the Novel Antiviral Properties of Naproxen against the Nucleoprotein of Influenza a Virus. Available online: https://journals.asm.org/doi/epdf/10.1128/AAC.02335-12?src=getftr (accessed on 24 March 2023).






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawełczyk, A.; Nowak, R.; Gazecka, M.; Jelińska, A.; Zaprutko, L.; Zmora, P. Novel Molecular Consortia of Cannabidiol with Nonsteroidal Anti-Inflammatory Drugs Inhibit Emerging Coronaviruses’ Entry. Pathogens 2023, 12, 951. https://doi.org/10.3390/pathogens12070951
Pawełczyk A, Nowak R, Gazecka M, Jelińska A, Zaprutko L, Zmora P. Novel Molecular Consortia of Cannabidiol with Nonsteroidal Anti-Inflammatory Drugs Inhibit Emerging Coronaviruses’ Entry. Pathogens. 2023; 12(7):951. https://doi.org/10.3390/pathogens12070951
Chicago/Turabian StylePawełczyk, Anna, Rafał Nowak, Monika Gazecka, Anna Jelińska, Lucjusz Zaprutko, and Paweł Zmora. 2023. "Novel Molecular Consortia of Cannabidiol with Nonsteroidal Anti-Inflammatory Drugs Inhibit Emerging Coronaviruses’ Entry" Pathogens 12, no. 7: 951. https://doi.org/10.3390/pathogens12070951
APA StylePawełczyk, A., Nowak, R., Gazecka, M., Jelińska, A., Zaprutko, L., & Zmora, P. (2023). Novel Molecular Consortia of Cannabidiol with Nonsteroidal Anti-Inflammatory Drugs Inhibit Emerging Coronaviruses’ Entry. Pathogens, 12(7), 951. https://doi.org/10.3390/pathogens12070951