Klebsiella pneumoniae—A Useful Pathogenic Strain for Biotechnological Purposes: Diols Biosynthesis under Controlled and Uncontrolled pH Levels
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
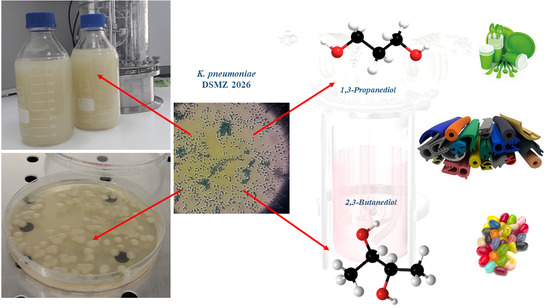
3.1. Microorganism and Culture Conditions
3.2. Batch Cultivation at Bioreactor Level
3.3. Testing Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castaneda, D.D.C.; Ramirez Duran, N.; Espinoza Rivera, I.; Marcela Caro Gonzalez, L.; Pablo Antonio Moreno Perez, M.; Mendieta Zeron, H. Atypical Klebsiella Species in a Third Level Hospital as Cause of Neonatal Infection. Jundishapur J. Microbiol. 2018, 11, e62393. [Google Scholar]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Lin, L.; Wu, Y.X.; Honhing, H.; He, P.F.; Li, G.Z.; He, P.B.; Xiong, G.R.; Yuan, Y.; He, Y.Q. Pathogenicity of Klebsiella pneumonia (KpC4) infecting maize and mice. J. Integr. Agric. 2016, 15, 1510–1520. [Google Scholar] [CrossRef] [Green Version]
- Aygun, F.; Aygun, F.D.; Varol, F.; Durak, C.; Çokuğraş, H.; Camcıoğlu, Y.; Çam, H. Infections with Carbapenem-Resistant Gram-Negative Bacteria are a Serious Problem Among Critically Ill Children: A Single-Centre Retrospective Study. Pathogens 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.H.; Rathnasingh, C.; Song, H.; Chung, B.W.; Lee, H.J.; Seung, D. Fermentation and evaluation of Klebsiella pneumoniae and K. oxytoca on the production of 2,3-butanediol. Bioprocess Biosyst. Eng. 2012, 35, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Kumar, V.; Ashok, S.; Song, H.; Rathnasingh, C.; Lee, H.J.; Seung, D.; Park, S. Isolation and characterization of the new Klebsiella pneumoniae J2B strain showing improved growth characteristics with reduced lipopolysaccharide formation. Bioprocess Biosyst. Eng. 2011, 16, 1134–1143. [Google Scholar] [CrossRef]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and Hospital-Acquired Klebsiella pneumoniae Urinary Tract Infections in Portugal: Virulence and Antibiotic Resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Russo, R.; Kolesnikova, I.; Kim, T.; Gupta, S.; Pericleous, A.; Kadouri, D.E.; Connell, N.D. Susceptibility of Virulent Yersinia pestis Bacteria to Predator Bacteria in the Lungs of Mice. Microorganisms 2018, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Podschun, R.; Ullmann, U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Benincasa, M.; Lagatolla, C.; Dolzani, L.; Milan, A.; Pacor, S.; Liut, G.; Tossi, A.; Cescutti, P.; Rizzo, R. Biofilms from Klebsiella pneumoniae: Matrix Polysaccharide Structure and Interactions with Antimicrobial Peptides. Microorganisms 2016, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Chhibber, S.; Gondil, V.; Kaur, J. Isolation, characterization, statistical optimization, and application of a novel broad-spectrum capsular depolymerase against Klebsiella pneumoniae from Bacillus siamensis SCVJ30. Biomed. Biotechnol. Res. J. 2018, 2, 125–131. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schembri, M.A.; Blom, J.; Krogfelt, K.A.; Klemm, P. Capsule and fimbria interaction in Klebsiella pneumoniae. Infect. Immun. 2005, 73, 4626–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorman, M.J.; Short, F.L. Klebsiella pneumoniae: When a colonizer turns bad. Nat. Rev. Microbiol. 2017, 15, 384. [Google Scholar] [CrossRef]
- Kumar, V.; Park, S. Potential and limitations of Klebsiella pneumoniae as a microbial cell factory utilizing glycerol as the carbon source. Biotechnol. Adv. 2018, 36, 150–167. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ferenczi, L.J.; Ștefănescu, B.E.; Vodnar, D.C. Inhibitory Potential of Lactobacillus plantarum on Escherichia coli. Bull. UASVM Food Sci. Technol. 2017, 74, 2. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Trif, M.; Cătoi, A.F.; Vodnar, D.C. Utilization of biodiesel derived-glycerol for 1,3-PD and citric acid production. Microb. Cell Fact. 2017, 16, 190. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.R.; Lee, S.M.; Heo, S.Y.; Seo, J.W.; Kim, C.H. Efficient production of 1,3-propanediol from crude glycerol by repeated fed-batch fermentation strategy of a lactate and 2,3-butanediol deficient mutant of Klebsiella pneumoniae. Microb. Cell Fact. 2018, 17, 92. [Google Scholar] [CrossRef]
- Parate, R.; Mane, R.; Dharne, M.; Rode, C. Mixed bacterial culture mediated direct conversion of bio-glycerol to diols. Bioresour. Technol. 2018, 250, 86–93. [Google Scholar] [CrossRef]
- Jung, S.G.; Jang, J.H.; Kim, A.Y.; Lim, M.C.; Kim, B.; Lee, J.; Kim, Y.R. Removal of pathogenic factors from 2,3-butanediol-producing Klebsiella species by inactivating virulence-related wabG gene. Appl. Microbiol. Biotechnol. 2013, 97, 1997–2007. [Google Scholar] [CrossRef]
- Böhme, K.; Fernández-No, I.C.; Barros-Velázquez, J.; Gallardo, J.M.; Cañas, B.; Calo-Mata, P. Rapid species identification of seafood spoilage and pathogenic Gram-positive bacteria by MALDI-TOF mass fingerprinting. Electrophoresis 2011, 32, 2951–2965. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J. Bacillus cereus, a Volatile Human Pathogen. Clin. Microbiol. Rev. 2010, 23, 382. [Google Scholar] [CrossRef] [Green Version]
- Forsberg, C.W. Production of 1,3-Propanediol from Glycerol by Clostridium acetobutylicum and Other Clostridium Species. Appl. Environ. Microbiol. 1987, 53, 639–643. [Google Scholar] [PubMed]
- Călinoiu, L.F.; Cătoi, A.F.; Vodnar, D.C. Solid-state yeast fermented wheat and oat bran as a route for delivery of antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Călinoiu, L.F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan coating applications in probiotic microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.F.; Vodnar, D.C. Screening of ten tomato varieties processing waste for bioactive components and their related antioxidant and antimicrobial activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Trif, M.; Vodnar, D.C.; Mitrea, L.; Rusu, A.V.; Socol, C.T. Design and development of oleoresins rich in carotenoids coated microbeads. Coatings 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Przystałowska, H.; Zeyland, J.; Szymanowska-Powałowska, D.; Szalata, M.; Słomski, R.; Lipiński, D.J.M. 1, 3-Propanediol production by new recombinant Escherichia coli containing genes from pathogenic bacteria. Microbiol. Res. 2015, 171, 1–7. [Google Scholar] [CrossRef]
- Celińska, E. Debottlenecking the 1,3-propanediol pathway by metabolic engineering. Biotechnol. Adv. 2010, 28, 519–530. [Google Scholar] [CrossRef]
- Tong, I.T.; Liao, H.H.; Cameron, D.C. 1,3-Propanediol production by Escherichia coli expressing genes from the Klebsiella pneumoniae dha regulon. Appl. Environ. Microbiol. 1991, 57, 3541–3546. [Google Scholar]
- Frommer, W.; Archer, L.; Boon, B.; Brunius, G.; Collins, C.H.; Crooy, P.; Doblhoff-Dier, O.; Donikian, R.; Economidis, J.; Frontali, C.; et al. Safe biotechnology (5). Recommendations for safe work with animal and human cell cultures concerning potential human pathogens. Appl. Microbiol. Biotechnol. 1993, 39, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Zhang, D.J.; Xu, Y.H.; Mu, Y.; Sun, Y.Q.; Xiu, Z.L. Microbial production of 1,3-propanediol from glycerol by Klebsiella pneumoniae under micro-aerobic conditions up to a pilot scale. Biotechnol. Lett. 2007, 29, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Călinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ștefănescu, B.E.; Pop, I.D.; Vodnar, D.C. Isolated Microorganisms for Bioconversion of Biodiesel-Derived Glycerol Into 1,3-Propanediol. Bull. UASVM Food Sci. Technol. 2017, 74, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Hazeena, S.H.; Nair Salini, C.; Sindhu, R.; Pandey, A.; Binod, P. Simultaneous saccharification and fermentation of oil palm front for the production of 2,3-butanediol. Bioresour. Technol. 2019, 278, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.Q.; Shen, J.T.; Yan, L.; Zhou, J.J.; Jiang, L.L.; Chen, Y.; Yuan, J.L.; Feng, E.; Xiu, Z.L. Advances in bioconversion of glycerol to 1,3-propanediol: Prospects and challenges. Process Biochem. 2018, 71, 134–146. [Google Scholar] [CrossRef]
- Vivek, N.; Pandey, A.; Binod, P. An efficient aqueous two phase systems using dual inorganic electrolytes to separate 1,3-propanediol from the fermented broth. Bioresour. Technol. 2018, 254, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.; Petrova, P. High production of 2,3-butanediol from glycerol by Klebsiella pneumoniae G31. Appl. Microbiol. Biotechnol. 2009, 84, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Biebl, H.; Zeng, A.P.; Menzel, K.; Deckwer, W.D. Fermentation of glycerol to 1,3-propanediol and 2,3-butanediol by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 1998, 50, 24–29. [Google Scholar] [CrossRef]
- Kumar, V.; Durgapal, M.; Sankaranarayanan, M.; Somasundar, A.; Rathnasingh, C.; Song, H.; Seung, D.; Park, S. Effects of mutation of 2,3-butanediol formation pathway on glycerol metabolism and 1,3-propanediol production by Klebsiella pneumoniae J2B. Bioresour. Technol. 2016, 214, 432–440. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiu, Z. Metabolic pathway analysis of glycerol metabolism in Klebsiella pneumoniae incorporating oxygen regulatory system. Biotechnol. Prog. 2009, 25, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Durgapal, M.; Kumar, V.; Yang, T.H.; Lee, H.J.; Seung, D.; Park, S. Production of 1,3-propanediol from glycerol using the newly isolated Klebsiella pneumoniae J2B. Bioresour. Technol. 2014, 159, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, D.; Segarra, S.; Montesinos, A.; Tortajada, M.; Ramon, D.; Rojas, A. Improved Raoultella planticola Strains for the Production of 2,3-Butanediol from Glycerol. Ferment. Basel 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Prada-Palomo, Y.; Romero-Vanegas, M.; Díaz-Ruíz, P.; Molina-Velasco, D.; Guzmán-Luna, C. Lactic Acid Production by Lactobacillus sp. From Biodiesel Derived Raw Glycerol. Cienc. Tecnol. Y Futuro 2012, 5, 57–65. [Google Scholar]
- Gao, C.; Yang, X.; Wang, H.; Rivero, C.P.; Li, C.; Cui, Z.; Qi, Q.; Lin, C.S.K. Robust succinic acid production from crude glycerol using engineered Yarrowia lipolytica. Biotechnol. Biofuels 2016, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.P.; de Lima, C.J.B.; Contiero, J. Production and productivity of 1-3 PD from glycerol by Klebsiella pneumoniae GLC29. Catal. Today 2015, 257, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Ma, B.; Xu, X.; Li, C.; Wang, L. Fast conversion of glycerol to 1,3-propanediol by a new strain of Klebsiella pneumoniae. Biochem. Eng. J. 2007, 37, 256–260. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.K.; Zhang, J.A.; Liu, D.H.; Sun, Y.; Liu, H.J.; Yang, M.D.; Xu, J.M. Pilot-scale production of 1,3-propanediol using Klebsiella pneumoniae. Process Biochem. 2007, 42, 740–744. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Chen, G.; Yan, S.J. Microbial production of 1,3-propanediol from glycerol by encapsulated Klebsiella pneumoniae. Biochem. Eng. J. 2006, 32, 93–99. [Google Scholar] [CrossRef]
- Evrard, B.; Balestrino, D.; Dosgilbert, A.; Bouya-Gachancard, J.L.J.; Charbonnel, N.; Forestier, C.; Tridon, A. Roles of Capsule and Lipopolysaccharide O Antigen in Interactions of Human Monocyte-Derived Dendritic Cells and Klebsiella pneumoniae. Infect. Immun. 2010, 78, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisse, S.; Passet, V.; Haugaard, A.B.; Babosan, A.; Kassis-Chikhani, N.; Struve, C.; Decre, D. wzi Gene Sequencing, a Rapid Method for Determination of Capsular Type for Klebsiella Strains. J. Clin. Microbiol. 2013, 51, 4073–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.H.; Huang, S.H.; Wu, C.C.; Liu, H.H.; Jinn, T.R.; Chen, Y.; Lin, C.T. Inhibition of Klebsiella pneumoniae Growth and Capsular Polysaccharide Biosynthesis by Fructus mume. J. Evid. Based Complementary Altern. Med. 2013, 2013, 621701. [Google Scholar]
- Menzel, K.; Zeng, A.P.; Biebl, H.; Deckwer, W.D. Kinetic, dynamic, and pathway studies of glycerol metabolism by Klebsiella pneumoniae in anaerobic continuous culture: 1. The phenomena and characterization of oscillation and hysteresis. Biotechnol. Bioeng. 1996, 52, 549–560. [Google Scholar] [CrossRef]
- Imbert, L.; Saussereau, E.; Lacroix, C. Analysis of Eight Glycols in Serum Using LC-ESI–MS-MS. J. Anal. Toxicol. 2014, 38, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Ranga, F.; Fetea, F.; Dulf, F.; Rusu, A.; Trif, M.; Vodnar, D.C. Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms 2019, 7, 265. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, N.R.; Halvorson, H.O. Application of Statistics to Problems in Bacteriology: IV. Experimental Comparison of the Dilution Method, the Plate Count, and the Direct Count for the Determination of Bacterial Populations. J. Bacteriol. 1935, 29, 609–634. [Google Scholar]
- Prakash. Methylene Blue Staining. Available online: https://www.protocols.io/view/Methylene-Blue-staining-fd7bi9n (accessed on 13 March 2019).



| Time (h) | pH | Substrate (glycerol) g/L | PDO g/L | BDO g/L | Viability log10 CFU/mL |
| 0 | 7.00 | 51.12 ± 0.02 | 0.00 ± 0.00 | 1.01 ± 0.00 | 8.91 ± 0.00 |
| 3 | 7.00 | 45.11 ± 3.21 | 8.86 ± 0.00 | 2.50 ± 0.00 | 9.37 ± 0.00 |
| 6 | 7.00 | 22.32 ± 2.00 | 16.59 ± 1.00 | 4.44 ± 0.02 | 9.33 ± 0.00 |
| 9 | 7.00 | 12.14 ± 0.98 | 21.22 ± 1.85 | 4.89 ± 0.09 | 9.61 ± 0.00 |
| 12 | 7.00 | 3.08 ± 0.02 | 22.89 ± 1.52 | 5.11 ± 0.10 | 9.64 ± 0.00 |
| 15 | 7.00 | 2.60 ± 0.19 | 27.51 ± 1.45 | 10.88 ± 0.92 | 9.62 ± 0.00 |
| 18 | 7.00 | 1.40 ± 0.02 | 30.63 ± 1.99 | 15.55 ± 1.03 | 9.34 ± 0.00 |
| 21 | 7.00 | 0.08 ± 0.00 | 30.03 ± 2.00 | 16.36 ± 1.00 | 8.47 ± 0.00 |
| 24 | 7.00 | 0.04 ± 0.00 | 28.63 ± 2.20 | 18.10 ± 1.10 | 8.42 ± 0.00 |
| Time (h) | pH | Substrate (glycerol) g/L | PDO g/L | BDO g/L | Viability log10 CFU/mL |
| 0 | 6.98 | 52.01 ± 0.00 | 0.00 ± 0.00 | 0.62 ± 0.00 | 8.30 ± 0.00 |
| 3 | 5.91 | 50.00 ± 0.00 | 3.08 ± 0.00 | 1.00 ± 0.00 | 8.18 ± 0.08 |
| 6 | 5.48 | 33.85 ± 0.00 | 7.00 ± 0.39 | 1.09 ± 0.00 | 8.42 ± 0.04 |
| 9 | 5.10 | 23.00 ± 0.10 | 9.52 ± 0.01 | 1.22 ± 0.00 | 8.42 ± 0.04 |
| 12 | 5.01 | 12.98 ± 0.58 | 9.80 ± 0.09 | 2.35 ± 0.21 | 8.53 ± 0.00 |
| 15 | 4.86 | 11.52 ± 0.88 | 11.83 ± 0.00 | 3.40 ± 0.21 | 8.51 ± 0.00 |
| 18 | 4.77 | 9.23 ± 0.07 | 12.45 ± 0.04 | 9.75 ± 0.14 | 8.33 ± 0.00 |
| 21 | 4.62 | 8.49 ± 0.04 | 12.01 ± 0.04 | 8.12 ± 0.43 | 8.26 ± 0.00 |
| 24 | 4.51 | 6.99 ± 0.06 | 11.08 ± 0.14 | 7.35 ± 0.00 | 7.04 ± 0.00 |
| Yield molProduct/molSubstrate | PDO | BDO | Lactic Acid | Citric Acid | Succinic Acid | References |
|---|---|---|---|---|---|---|
| Maximum theoretical yield | 0.72 | 0.63 | 0.73 | 0.51 | 0.50 | [17,41,42,43,44,45] |
| Controlled pH | 0.68 | 0.35 | 0.00 | 0.02 | 0.03 | This study |
| Uncontrolled pH | 0.25 | 0.14 | 0.00 | 0.01 | 0.03 | This study |
| Controlled pH | ||||
| Time (h) | pH | Lactic Acid g/L | Citric Acid g/L | Succinic Acid g/L |
| 0 | 7.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.04 ± 0.00 |
| 12 | 7.00 | 0.00 ± 0.00 | 2.63 ± 0.02 | 1.96 ± 0.05 |
| 24 | 7.00 | 0.00 ± 0.00 | 2.67 ± 0.04 | 2.09 ± 0.05 |
| Uncontrolled pH | ||||
| Time (h) | pH | Lactic Acid g/L | Citric Acid g/L | Succinic Acid g/L |
| 0 | 6.98 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.16 ± 0.00 |
| 12 | 5.01 | 0.00 ± 0.00 | 1.11 ± 0.00 | 2.18 ± 0.02 |
| 24 | 4.51 | 0.00 ± 0.00 | 2.23 ± 0.052 | 2.35 ± 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrea, L.; Vodnar, D.C. Klebsiella pneumoniae—A Useful Pathogenic Strain for Biotechnological Purposes: Diols Biosynthesis under Controlled and Uncontrolled pH Levels. Pathogens 2019, 8, 293. https://doi.org/10.3390/pathogens8040293
Mitrea L, Vodnar DC. Klebsiella pneumoniae—A Useful Pathogenic Strain for Biotechnological Purposes: Diols Biosynthesis under Controlled and Uncontrolled pH Levels. Pathogens. 2019; 8(4):293. https://doi.org/10.3390/pathogens8040293
Chicago/Turabian StyleMitrea, Laura, and Dan Cristian Vodnar. 2019. "Klebsiella pneumoniae—A Useful Pathogenic Strain for Biotechnological Purposes: Diols Biosynthesis under Controlled and Uncontrolled pH Levels" Pathogens 8, no. 4: 293. https://doi.org/10.3390/pathogens8040293
APA StyleMitrea, L., & Vodnar, D. C. (2019). Klebsiella pneumoniae—A Useful Pathogenic Strain for Biotechnological Purposes: Diols Biosynthesis under Controlled and Uncontrolled pH Levels. Pathogens, 8(4), 293. https://doi.org/10.3390/pathogens8040293