Integrative Approach to Phlebotomus mascittii Grassi, 1908: First Record in Vienna with New Morphological and Molecular Insights
Abstract
:1. Introduction
2. Results
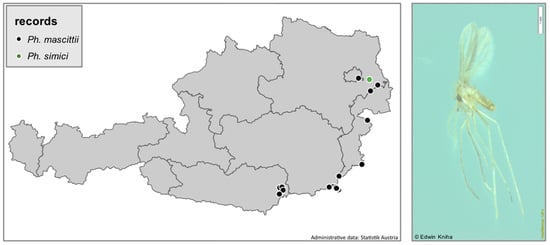
2.1. Sand Fly Trapping and Identification
2.2. Pairwise Distances of Transphlebotomus Species
2.3. Haplotype Analysis of Ph. Mascittii from Austria and Other Countries
2.4. Climatic Parameters
3. Discussion
4. Material and Methods
4.1. Sand Fly Trapping and Available Material
4.2. Climate Data
4.3. Mapping of Sand Fly Distribution
4.4. Morphological Identification
4.5. Molecular Identification by PCR and Sequencing
4.6. Identification by MALDI-TOF MS Protein Profiling
4.7. Leishmania spp. Screening
4.8. Sequence Analyses
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Biology of Phlebotomine Sand Flies as Vectors of Disease Agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Naucke, T.J.; Pesson, B. Presence of Phlebotomus (Transphlebotomus) mascittii Grassi, 1908 (Diptera: Psychodidae) in Germany. Parasitol. Res. 2000, 86, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Naucke, T.J.; Schmitt, C. Is leishmaniasis becoming endemic in Germany? Int. J. Med. Microbiol. 2004, 293, 179–181. [Google Scholar] [CrossRef]
- Naucke, T.J.; Lorentz, S.; Rauchenwald, F.; Aspöck, H. Phlebotomus (Transphlebotomus) mascittii Grassi, 1908, in Carinthia: First record of the occurrence of sandflies in Austria (Diptera: Psychodidae: Phlebotominae). Parasitol. Res. 2011, 109, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Poeppl, W.; Obwaller, A.G.; Weiler, M.; Burgmann, H.; Mooseder, G.; Lorentz, S.; Rauchenwald, F.; Aspöck, H.; Walochnik, J.; Naucke, T.J. Emergence of sandflies (Phlebotominae) in Austria, a Central European country. Parasitol. Res. 2013, 112, 4231–4237. [Google Scholar] [CrossRef] [Green Version]
- Obwaller, A.G.; Poeppl, W.; Naucke, T.J.; Luksch, U.; Mooseder, G.; Aspöck, H.; Walochnik, J. Stable populations of sandflies ( Phlebotominae ) in Eastern Austria: A comparison of the trapping seasons 2012 and 2013. Trends Entomol. 2014, 2, 1–5. [Google Scholar]
- Dvořák, V.; Hlavackova, K.; Kocisova, A.; Volf, P. First record of Phlebotomus (Transphlebotomus) mascittii in Slovakia. Parasite 2016, 23, 48. [Google Scholar] [CrossRef] [Green Version]
- Kniha, E.; Dvořák, V.; Milchram, M.; Obwaller, A.G.; Koehsler, M.; Poeppl, W.; Antoniou, M.; Chaskopoulou, A.; Paronyan, L.; Stefanovska, J.; et al. Phlebotomus (Adlerius) simici Nitzulescu, 1931: First record in Austria and phylogenetic relationship with other Adlerius species. Parasit. Vectors 2020, (in press). [Google Scholar]
- Aransay, A.M.; Testa, J.M.; Morillas-Márquez, F.; Lucientes, J.; Ready, P.D. Distribution of sandfly species in relation to canine leishmaniasis from the Ebro Valley to Valencia, northeastern Spain. Parasitol. Res. 2004, 94, 416–420. [Google Scholar] [CrossRef]
- Depaquit, J.; Naucke, T.J.; Schmitt, C.; Ferté, H.; Léger, N. A molecular analysis of the subgenus Transphlebotomus Artemiev, 1984 (Phlebotomus, Diptera, Psychodidae) inferred from ND4 mtDNA with new northern records of Phlebotomus mascittii Grassi, 1908. Parasitol. Res. 2005, 95, 113–116. [Google Scholar] [CrossRef]
- Veronesi, E.; Pilani, R.; Carrieri, M.; Bellini, R. Trapping sand flies (Diptera: Psychodidae) in the Emilia-Romagna region of northern Italy. J. Vector Ecol. 2007, 32, 313–318. [Google Scholar] [CrossRef]
- Naucke, T.J.; Menn, B.; Massberg, D.; Lorentz, S. Winter activity of Phlebotomus (Transphlebotomus) mascittii, Grassi 1908 (Diptera: Psychodidae) on the island of Corsica. Parasitol. Res. 2008, 103, 477–479. [Google Scholar] [CrossRef]
- Bosnić, S.; Gradoni, L.; Khoury, C.; Maroli, M. A review of leishmaniasis in Dalmatia (Croatia) and results from recent surveys on phlebotomine sandflies in three southern counties. Acta Trop. 2006, 99, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Grimm, F.; Gessler, M.; Jenni, L. Aspects of sandfly biology in southern Switzerland. Med. Vet. Entomol. 1993, 7, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Praprotnik, E.; Zupan, S.; Ivović, V. Morphological and Molecular Identification of Phlebotomus mascittii Grassi, 1908 Populations From Slovenia. J. Med. Entomol. 2019, 56, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Vaselek, S.; Dvořák, V.; Hlavackova, K.; Ayhan, N.; Halada, P.; Oguz, G.; Ivović, V.; Ozbel, Y.; Charrel, R.N.; Alten, B.; et al. A survey of sand flies (Diptera, Phlebotominae) along recurrent transit routes in Serbia. Acta Trop. 2019, 197, 105063. [Google Scholar] [CrossRef] [PubMed]
- Farkas, R.; Tánczos, B.; Bongiorno, G.; Maroli, M.; Dereure, J.; Ready, P.D. First Surveys to Investigate the Presence of Canine Leishmaniasis and Its Phlebotomine Vectors in Hungary. Vector-Borne Zoonotic Dis. 2011, 11, 823–834. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Tarallo, V.D.; Latrofa, M.S.; Falchi, A.; Lia, R.P.; Otranto, D. Ecology of phlebotomine sand flies and Leishmania infantum infection in a rural area of southern Italy. Acta Trop. 2014, 137, 67–73. [Google Scholar] [CrossRef]
- Berdjane-Brouk, Z.; Charrel, R.N.; Bitam, I.; Hamrioui, B.; Izri, A. Record of Phlebotomus (Transphlebotomus) mascittii Grassi, 1908 and Phlebotomus (Laroussius) chadli Rioux, Juminer & Gibily, 1966 female in Algeria. Parasite 2011, 18, 337–339. [Google Scholar]
- Melaun, C.; Krüger, A.; Werblow, A.; Klimpel, S. New record of the suspected leishmaniasis vector Phlebotomus (Transphlebotomus) mascittii Grassi, 1908 (Diptera: Psychodidae: Phlebotominae)—The northernmost phlebotomine sandfly occurrence in the Palearctic region. Parasitol. Res. 2014, 113, 2295–2301. [Google Scholar] [CrossRef]
- Obwaller, A.G.; Karakus, M.; Poeppl, W.; Töz, S.; Özbel, Y.; Aspöck, H.; Walochnik, J. Could Phlebotomus mascittii play a role as a natural vector for Leishmania infantum? New data. Parasit. Vectors 2016, 9, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanet, S.; Sposimo, P.; Trisciuoglio, A.; Giannini, F.; Strumia, F.; Ferroglio, E. Epidemiology of Leishmania infantum, Toxoplasma gondii, and Neospora caninum in Rattus rattus in absence of domestic reservoir and definitive hosts. Vet. Parasitol. 2014, 199, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Schönian, G.; Bañuls, A.L.; Hide, M.; Pratlong, F.; Lorenz, E.; Röllinghoff, M.; Mertens, R. Visceral leishmaniasis in a German child who had never entered a known endemic area: Case report and review of the literature. Clin. Infect. Dis. 2001, 32, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Stechele, M.; Hetzel, U.; Domingo, M.; Schönian, G.; Zahner, H.; Burkhardt, E. Cutaneous leishmaniosis in a horse in southern Germany caused by Leishmania infantum. Vet. Parasitol. 2002, 109, 9–17. [Google Scholar] [CrossRef]
- Kollaritsch, H.; Emminger, W.; Zaunschirm, A.; Aspöck, H. Suspected Autochthonous Kala-azar in Austria. Lancet 1989, 1, 901–902. [Google Scholar] [CrossRef]
- Beyreder, J. Ein Fall von Leishmaniose in Niederösterreich. Wien. Med. Wochenschr. 1962, 115, 900–901. [Google Scholar]
- Kasap, O.E.; Dvořák, V.; Depaquit, J.; Alten, B.; Votypka, J.; Volf, P. Phylogeography of the subgenus Transphlebotomus Artemiev with description of two new species, Phlebotomus anatolicus n. sp. and Phlebotomus killicki n. sp. Infect. Genet. Evol. 2015, 34, 467–479. [Google Scholar] [CrossRef]
- Lafri, I.; Almeras, L.; Bitam, I.; Caputo, A.; Yssouf, A.; Forestier, C.L.; Izri, A.; Raoult, D.; Parola, P. Identification of Algerian Field-Caught Phlebotomine Sand Fly Vectors by MALDI-TOF MS. PLoS Negl. Trop. Dis. 2016, 10, e0004351. [Google Scholar] [CrossRef]
- Pareyn, M.; Dvořák, V.; Halada, P.; Van Houtte, N.; Medhin, G.; de Kesel, W.; Merdekios, B.; Massebo, F.; Leirs, H.; Volf, P. An integrative approach to identify sand fly vectors of leishmaniases in Ethiopia by morphological and molecular techniques. Parasit. Vectors 2020, 13, 580. [Google Scholar] [CrossRef]
- Oerther, S.; Jöst, H.; Heitmann, A.; Lühken, R.; Krüger, A.; Steinhausen, I.; Brinker, C.; Lorentz, S.; Marx, M.; Schmidt-Chanasit, J.; et al. Phlebotomine sand flies in Southwest Germany: An update with records in new locations. Parasit. Vectors 2020, 13, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, E.; Bongiorno, G.; Ciolli, E.; Di Muccio, T.; Scalone, A.; Gramiccia, M.; Gradoni, L.; Maroli, M. Seasonal phenology, host-blood feeding preferences and natural Leishmania infection of Phlebotomus perniciosus (Diptera, Psychodidae) in a high-endemic focus of canine leishmaniasis in Rome province, Italy. Acta Trop. 2008, 105, 158–165. [Google Scholar] [CrossRef]
- Ivović, V.; Kalan, K.; Zupan, S.; Bužan, E. Illegal waste sites as a potential micro foci of Mediterranean Leishmaniasis: First records of phlebotomine sand flies (Diptera: Psychodidae) from Slovenia. Acta Vet. Brno 2015, 65, 348–357. [Google Scholar]
- Trájer, A.J.; Sebestyén, V. The changing distribution of Leishmania infantum Nicolle, 1908 and its Mediterranean sandfly vectors in the last 140 kys. Sci. Rep. 2019, 9, 11820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simsek, F.M.; Alten, B.; Caglar, S.S.; Ozbep, Y.; Aytekin, A.M.; Kaynas, S.; Belen, A.; Kasap, O.E.; Yaman, M.; Rastgeldi, S. Distribution and altitudinal structuring of phlebotomine sand flies (Diptera: Psychodidae) in southern Anatolia, Turkey: Their relation to human cutaneous leishmaniasis. J. Vector Ecol. 2007, 32, 269–279. [Google Scholar] [CrossRef]
- Tichy, H.; Kallina, W. Insect hygroreceptor responses to continuous changes in humidity and air pressure. J. Neurophysiol. 2010, 103, 3274–3286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leschnik, M.; Löwenstein, M.; Edelhofer, R.; Kirtz, G. Imported non-endemic, arthropod-borne and parasitic infectious diseases in Austrian dogs. Wien. Klin. Wochenschr. 2008, 120, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Aspöck, H.; Walochnik, J. When sandflies move north. Public Health 2009, 20, 24–31. [Google Scholar]
- Aspöck, H. Postglacial formation and fluctuations of the biodiversity of Central Europe in the light of climate change. Parasitol. Res. 2008, 103, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Depaquit, J. Molecular systematics applied to Phlebotomine sandflies: Review and perspectives. Infect. Genet. Evol. 2014, 28, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Depaquit, J.; Ferté, H.; Léger, N.; Lefranc, F.; Alves-Pires, C.; Hanafi, H.; Maroli, M.; Morillas-Márquez, F.; Rioux, J.A.; Svobodova, M.; et al. ITS 2 sequences heterogeneity in Phlebotomus sergenti and Phlebotomus similis (Diptera, Psychodidae): Possible consequences in their ability to transmit Leishmania tropica. Int. J. Parasitol. 2002, 32, 1123–1131. [Google Scholar] [CrossRef]
- QGIS Development Team QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 29 October 2019).
- Lewis, D.J. A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae). Bull. Br. Mus. Nat. Hist. 1982, 45, 121–209. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, V.; Halada, P.; Hlacvackiva, K.; Dokianakis, E.; Antoniou, M.; Volf, P. Identification of phlebotomine sand flies (Diptera: Psychodidae) by matrix-assisted laser desorption/ionization time of flight mass spectrometry. Parasit. Vectors 2014, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Halada, P.; Hlavackova, K.; Risueño, J.; Berriatua, E.; Volf, P.; Dvořák, V. Effect of trapping method on species identification of phlebotomine sandflies by MALDI-TOF MS protein profiling. Med. Vet. Entomol. 2018, 32, 388–392. [Google Scholar] [CrossRef]
- Dvořák, V.; Tsirigotakis, N.; Pavlou, C.; Dokianakis, E.; Akhoundi, M.; Halada, P.; Volf, P.; Depaquit, J.; Antoniou, M. Sand fly fauna of Crete and the description of Phlebotomus (Adlerius) creticus n. sp. Parasit. Vectors 2020, 13, 547. [Google Scholar] [CrossRef]
- El Tai, N.O.; Osman, F.O.; El Far, M.; Presber, W.; Schönian, G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 575–579. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Bandelt, H.-J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 5 May 2020).






| Id | Location (Federal State) | Latitude, Longitude | Altitude (m.a.s.l.) | Trapping Site (Potential Host) a | Specimen (Male/Female) | Species | Trap Date b | Traps Set (Type) |
|---|---|---|---|---|---|---|---|---|
| 47 | Kaiser-steinbruch (Burgenland) | 47.9911, 16.7103 | 179 m | outside, dog pound (dog) | 1 (0m/1f) | Ph. mascittii | 11.07.13 | 1 (CDC light) |
| 1 (0m/1f) | Ph. mascittii | 26.07.13 | 1 (CDC light) | |||||
| 64 | Neuhaus/Klausenbach (Burgenland) | 46.8683, 16.0232 | 274 m | outside, human dwelling (rodents possibly) | 1 (0m/1f) | Ph. mascittii | 30.07.19 | 1 (BG sentinel & CO2) |
| 1 (0m/1f) | Ph. mascittii | 25.08.19 | 1 (BG sentinel & CO2) | |||||
| 16 | Neu Albern (Vienna) | 48.1676, 16.4801 | 157 m | outside, roofed shed with hay (horse, dog) | 1 (1m/0f) | Ph. mascittii | 17.07.19 | 1 (CDC light & dry ice) |
| 25 | Laafeld (Styria) | 46.6867, 16.0069 | 207 m | outside, chicken shed (chicken, geese, deer) | 1 (0m/1f) | Ph. mascittii | 06.08.18 | 1 (CDC light & dry ice) |
| 2 (0m/2f) | Ph. mascittii | 05.08.19 | 1 (CDC light & dry ice) | |||||
| 48 | Hummers-dorf (Styria) | 46.7076, 15.9812 | 209 m | inside and outside, barn (chicken, dog) | 6 (0m/6f) | Ph. mascittii | 05.07.15 | 1 (CDC light) |
| 8 (1m/7f) | Ph. mascittii | 06.07.15 | 2 (CDC light) | |||||
| 49 | Bad Radkersburg (Styria) | 46.7012, 15.9756 | 209 m | inside, old chicken shed (no) | 2 (0m/2f) | Ph. mascittii | 06.07.15 | 1 (CDC light) |
| 54 | Unterpurkla (Styria) | 46.7319, 15.9062 | 223 m | inside, barn (chicken, cat) | 4(0m/4f) | Ph. mascittii | 06.07.15 | 1 (CDC light) |
| Species | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| 1 | Phlebotomus mascittii | 0.09/0.3 | ||||
| 2 | Phlebotomus canaaniticus | 10.7/14.7 | −/− a | |||
| 3 | Phlebotomus anatolicus | 10.9/13.9 | 6.6/8.7 | 0.2/0.2 | ||
| 4 | Phlebotomus killicki | 12.2/12.9 | 11.4/13.7 | 12.1/12.7 | 0.6/1.4 | |
| 5 | Phlebotomus economidesi | 15.4/13.8 | 13.2/13.3 | 14.5/12.8 | 13.4/9.9 | − a/1.9 |
| COI | cytb | ||||
|---|---|---|---|---|---|
| Country, Location | GenBank | Haplotype | GenBank | Haplotype | Reference |
| France, Cévennes | - | - | KR336654.1 | Cytb_2 | Kasap et al. (2015) |
| Corsica, Porto Vecchio 1 | - | - | KR336655.1 | Cytb_3 | Kasap et al. (2015) |
| Corsica, Porto Vecchio 2 | - | - | KR336656.1 | Cytb_1 | Kasap et al. (2015) |
| Belgium, Saint-Cécile | - | - | KR336656.1 | Cytb_1 | Kasap et al. (2015) |
| Germany, Neuenburg | - | - | KR336656.1 | Cytb_1 | Kasap et al. (2015) |
| France, Haute-Pyrénées | - | - | HQ023281.1 | Cytb_1 | Mahamdallie et al. (2011) |
| Serbia, Vojdovina | KY848831.1 | COI_1 | - | - | Vaselek et al. (2017) |
| Serbia, Krasava | MN003381.1 | COI_2 | MK991774.1 | Cytb_1 | Vaselek et al. (2019) |
| Slovakia, Pernek | KX963380.1 | COI_1 | - | - | Dvořák et al. (2016) |
| Slovenia, Truske | - | - | MG800323.1 | Cytb_4 | Praprotnik et al. (2019) |
| Slovenia, Cetore | KX981916.1 | COI_2 | MG800324.1 | Cytb_5 | Hlavackova (GenBank)/Praprotnik et al. (2019) |
| Slovenia, Velike Zablje | KX869078.1 | COI_2 | - | - | Hlavackova (GenBank) |
| Austria, Burgenland, Kaisersteinbruch | MN812827.1 | COI_2 | MN812832.1 | Cytb_1 | present study |
| Austria, Burgenland, Neuhaus/Klausen | MN812828.1 | COI_2 | MN812833.1 | Cytb_1 | present study |
| Austria, Vienna | MN812829.1 | COI_1 | MN812834.1 | Cytb_1 | present study |
| Austria, Styria, Laafeld | MN812830.1 | COI_2 | MN812835.1 | Cytb_1 | present study |
| Austria, Styria, Hummersdorf | MT332686.1 | COI_1 | MT332689.1 | Cytb_1 | present study |
| Austria, Styria, Bad Radkersburg | MT332687.1 | COI_1 | MT332690.1 | Cytb_1 | present study |
| Austria, Styria, Unterpurkla | MT332688.1 | COI_2 | MT332691.1 | Cytb_1 | present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kniha, E.; Dvořák, V.; Halada, P.; Milchram, M.; Obwaller, A.G.; Kuhls, K.; Schlegel, S.; Köhsler, M.; Poeppl, W.; Bakran-Lebl, K.; et al. Integrative Approach to Phlebotomus mascittii Grassi, 1908: First Record in Vienna with New Morphological and Molecular Insights. Pathogens 2020, 9, 1032. https://doi.org/10.3390/pathogens9121032
Kniha E, Dvořák V, Halada P, Milchram M, Obwaller AG, Kuhls K, Schlegel S, Köhsler M, Poeppl W, Bakran-Lebl K, et al. Integrative Approach to Phlebotomus mascittii Grassi, 1908: First Record in Vienna with New Morphological and Molecular Insights. Pathogens. 2020; 9(12):1032. https://doi.org/10.3390/pathogens9121032
Chicago/Turabian StyleKniha, Edwin, Vít Dvořák, Petr Halada, Markus Milchram, Adelheid G. Obwaller, Katrin Kuhls, Susanne Schlegel, Martina Köhsler, Wolfgang Poeppl, Karin Bakran-Lebl, and et al. 2020. "Integrative Approach to Phlebotomus mascittii Grassi, 1908: First Record in Vienna with New Morphological and Molecular Insights" Pathogens 9, no. 12: 1032. https://doi.org/10.3390/pathogens9121032
APA StyleKniha, E., Dvořák, V., Halada, P., Milchram, M., Obwaller, A. G., Kuhls, K., Schlegel, S., Köhsler, M., Poeppl, W., Bakran-Lebl, K., Fuehrer, H. -P., Volfová, V., Mooseder, G., Ivovic, V., Volf, P., & Walochnik, J. (2020). Integrative Approach to Phlebotomus mascittii Grassi, 1908: First Record in Vienna with New Morphological and Molecular Insights. Pathogens, 9(12), 1032. https://doi.org/10.3390/pathogens9121032