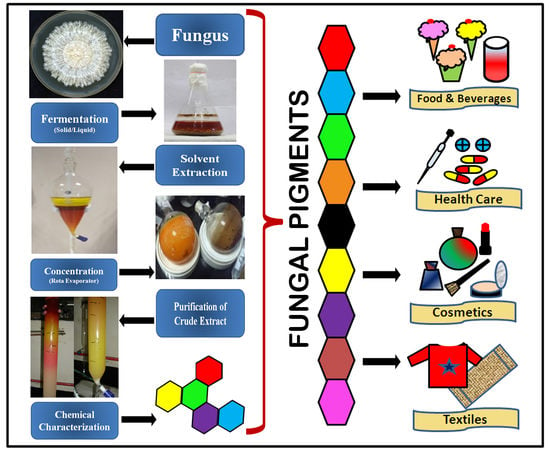
Fungal Pigments and Their Prospects in Different Industries
Abstract
:1. Introduction
2. Natural Pigments
3. Fungal Pigments
4. Optimization for Enhancement of Pigment Production
5. Applications or Biological Activities of Fungal Pigments
5.1. Fungal Pigments as Food Colorants
5.2. Fungal Pigments as Antimicrobial Agents
5.3. Fungal Pigments as Antioxidant Agents
5.4. Fungal Pigments as Cytotoxic Agents
5.5. Fungal Pigments as Anticancer Agents
5.6. Fungal Pigments in the Cosmetic Industry
5.7. Fungal Pigments in the Textile Industry
5.8. Fungal Pigments in Dyeing Woods or as Color Modifiers
5.9. Fungal Pigments in (Opto) Electronics
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, M.P.N.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide application. Front. Microbiol. 2017, 8, 1113. [Google Scholar]
- Downham, A.; Collins, P. Coloring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Osman, M.Y.; Sharaf, I.A.; Osman, H.M.Y.; El-Khouly, Z.A.; Ahmed, E.I. Synthetic organic food coloring agents and their degraded products: Effects on human and rat cholinesterases. Br. J. Biomed. Sci. 2004, 61, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Babitha, S. Microbial pigments. In Biotechnology for Agro-Industrial Residues Utilization; Nigam, P.S., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 147–162. [Google Scholar]
- Samanta, A.K.; Agarwal, P. Application of natural dyes on textiles. Indian J. Fibre Text. Res. 2009, 34, 384–399. [Google Scholar]
- Ratna, P.B.S. Pollution due to synthetic dyes toxicity and carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940–955. [Google Scholar]
- Arora, S. Textile dyes: Its impact on the environment and its treatment. J. Bioremediat. Biodegrad. 2014, 5, 1. [Google Scholar] [CrossRef]
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Chatterjee, S.; Sen, S.K. Biotechnological potential of natural food grade biocolorants. Afr. J. Biotechnol. 2008, 7, 2972–2985. [Google Scholar]
- Joshi, V.K.; Attri, D.; Bala, A.; Bhushan, S. Microbial pigments. Indian J. Biotechnol. 2003, 2, 362–369. [Google Scholar]
- Aberoumand, A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J. Dairy Food Sci. 2011, 6, 71–78. [Google Scholar]
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–321. [Google Scholar]
- Latha, B.V.; Jeevaratnam, K. Purification and characterization of the pigments from Rhodotorula glutinis DFR-PDY isolated from a natural source. Glob. J. Biotechnol. Biochem. 2010, 5, 166–174. [Google Scholar]
- Nagpal, N.; Munjal, N.; Chatterjee, S. Microbial pigments with health benefits—A mini review. Trends Biosci. 2011, 4, 157–160. [Google Scholar]
- Ahmad, W.A.; Ahmad, W.Y.W.; Zakaria, Z.A.; Yusof, N.Z. Isolation of pigment-producing bacteria and characterization of the extracted pigments. In Application of Bacterial Pigments as a Colorant; SpringerBriefs in Molecular Science; Springer: Berlin/Heidelberg, Germany, 2012; pp. 25–44. [Google Scholar]
- Kirti, K.; Amita, S.; Priti, S.; Kumar, A.M.; Jyoti, S. Colorful world of microbes: Carotenoids and their applications. Adv. Biol. 2014, 1, 1–13. [Google Scholar] [CrossRef]
- Mata-Gomez, L.C.; Montanez, J.C.; Mendez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef]
- Kumar, A.; Vishwakarma, H.S.; Singh, J.; Dwivedi, S.; Kumar, M. Microbial pigments: Production and their applications in various industries. Int. J. Phram. Chem. Biol. Sci. 2015, 5, 203–212. [Google Scholar]
- Sarkar, S.L.; Saha, P.; Sultana, N.; Akter, S. Exploring textile dye from microorganisms, an eco-friendly alternative. Microbiol. Res. J. Int. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms 2019, 7, 186. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process. Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D.; Gupta, S. Natural useful therapeutic products from microbes. J. Microbiol. Exp. 2014, 1, 30–37. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.U.; Khan, A.L.; Shinwari, Z.K.; Al-Harrasi, A. Therapeutic applications of bacterial pigments: A review of current status and future opportunities. 3 Biotech 2018, 8, 207. [Google Scholar] [CrossRef]
- Indra Arulselvi, P.; Umamaheswari, S.; Ranandkumar Sharma, G.; Karthik, C.; Jayakrishna, C. Screening of yellow pigment producing bacterial isolates from various eco-climatic areas and analysis of the carotenoid produced by the isolate. J. Food Process. Technol. 2014, 5, 292. [Google Scholar]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and colorants from filamentous fungi. In Fungal Metabolites; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 499–568. [Google Scholar]
- Gmoser, R.; Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol. Biotechnol. 2017, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Manikprabhu, D.; Lingappa, K. γ Actinorhodin a natural and attorney source for the synthetic dye to detect acid production of fungi. Saudi J. Biol. Sci. 2013, 20, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, R.A.; Wilmering, A.M.; Baumeister, M. The use of green-stained wood caused by the fungus Chlorociboria in intarsia masterpieces from the 15th century. Holzforsch Int. J. Biol. Chem. Phys. Technol. Wood 1992, 46, 225–232. [Google Scholar]
- Butler, M.J.; Day, A.W. Fungal melanins: A review. Can. J. Microbiol. 1998, 44, 1115–1136. [Google Scholar] [CrossRef]
- Sakaki, T.; Shibata, M.; Mukai, K.; Sakai, M.; Wakamatsu, K.; Miyauchi, S. Chlorociboria aeruginosa pigment as algicide. Jpn. Kokai Tokkyo Koho JP 2002, 2002291493. [Google Scholar]
- Carvalho, J.C.; Pandey, A.; Babitha, S.; Soccol, C.R. Production of Monascus biopigments: An overview. Agro Food Ind. Hi-Tech 2003, 14, 37–42. [Google Scholar]
- Feng, Y.; Shao, Y.; Chen, F. Monascus pigments. J. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440. [Google Scholar] [CrossRef]
- Robinson, S.C.; Tudor, D.; Snider, H.; Cooper, P.A. Stimulating growth and xylindein production of Chlorociboria aeruginascens in agar-based systems. AMB Express 2012, 2, 15. [Google Scholar] [CrossRef]
- Tudor, D. Fungal Pigment Formation in Wood Substrate. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2013. [Google Scholar]
- Tudor, D.; Robinson, S.C.; Cooper, P.A. The influence of pH on pigment formation by lignicolous fungi. Int. Biodeterior. Biodegrad. 2013, 80, 22–28. [Google Scholar] [CrossRef]
- Robinson, S.C.; Tudor, D.; Zhang, W.R.; Ng, S.; Cooper, P.A. Ability of three yellow pigment producing fungi to colour wood under controlled conditions. Int. Wood Prod. J. 2014, 5, 103–107. [Google Scholar] [CrossRef]
- Hinsch, E.M.; Chen, H.L.; Weber, G.; Robinson, S.C. Colorfastness of extracted wood-staining fungal pigments on fabrics: A new potential for textile dyes. J. Text. Appar. Technol. Manag. 2015, 9, 1–11. [Google Scholar]
- Tam, W.T.E.; Tsang, C.C.; Lau, K.P.S.; Woo, C.Y.P. Polyketides, toxins, and pigments in Penicillium marneffei. Toxins 2015, 7, 4421–4436. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, V.A.; Galleguillos, F.; Robinson, S. Fungal pigments from spalting fungi attenuating blue stain in Pinus spp. Int. Biodeterior. Biodegrad. 2016, 107, 154–157. [Google Scholar] [CrossRef]
- Robinson, S.C.; Michaelsen, H.; Robinson, J.C. Spalted Wood, History, Science and Art of an Unique Material; Schiffer Publishing: Atglen, PA, USA, 2016; pp. 1–288. [Google Scholar]
- Souza, P.N.C.; Grigoletto, T.L.B.; Moraes, L.A.B.; Abreu, L.M.; Souza, L.H.; Santos, C.; Galvao, L.R.; Cardoso, P.G. Production and chemical characterization of pigments in filamentous fungi. Microbiology 2016, 162, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Agurto, M.E.P.; Gutierrez, S.M.V.; Chen, H.-L.; Robinson, S.C. Wood-rotting fungal pigments as colorant coatings on oil-based textile dyes. Coatings 2017, 7, 152. [Google Scholar] [CrossRef]
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodriguez-Ortiz, R.; Hornero-Mendez, D.; Limon, M.C. Carotenoid biosynthesis in Fusarium. J. Fungi 2017, 3, 39. [Google Scholar] [CrossRef]
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.; Lisboa, H.F.; Gonçalves, R.C. Production of melanin pigment by fungi and its biotechnological applications. In Melanin; Blumenberg, M., Ed.; InTechOpen: London, UK, 2017; pp. 45–75. [Google Scholar]
- Vega Gutierrez, P.; Robinson, S. Determining the presence of spalted wood in spanish marquetry woodworks of the 1500s through the 1800s. Coatings 2017, 7, 188. [Google Scholar] [CrossRef]
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Nagia, F.A.; El-Mohamedy, R.S.R. Dyeing of wool with natural anthraquinone dyes from Fusarium oxysporum. Dyes Pigm. 2007, 75, 550–555. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Thrane, A.U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010, 28, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.S.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Went, F.A.F.C. Monascus purpureus le champignon de l’ang-quac une nouvelle thelebolee. Ann. Des. Sci. Nat. Bot. Biol. Veg. 1895, 8, 1–18. [Google Scholar]
- Fabre, C.E.; Santerre, A.L.; Loret, M.O.; Baberian, R.; Pareilleux, A.; Goma, G.; Blanc, P.J. Production and food applications of the red pigments of Monascus ruber. J. Food Sci. 1993, 58, 1099–1102. [Google Scholar] [CrossRef]
- Hajjaj, H.; Klaebe, A.; Loret, M.O.; Tzedakis, T.; Goma, G.; Blanc, P.J. Production and identification of N-glucosylrubropunctamine and N-glucosylmonascorubramine from Monascus ruber and occurrence of electron donor-acceptor complexes in these red pigments. Appl. Environ. Microbiol. 1997, 63, 2671–2678. [Google Scholar]
- Lian, X.; Wang, C.; Guo, K. Identification of new red pigments produced by Monascus ruber. Dyes Pigm. 2007, 73, 121–125. [Google Scholar] [CrossRef]
- Loret, M.O.; Morel, S. Isolation and structural characterization of two new metabolites from Monascus. J. Agric. Food Chem. 2010, 58, 1800–1803. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, O.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-W.; Hsu, L.-C.; Liang, Y.-H.; Kuo, Y.-H.; Pan, T.-M. Monaphilones A–C, three new antiproliferative azaphilone derivatives from Monascus purpureus NTU 568. J. Agric. Food Chem. 2010, 58, 8211–8216. [Google Scholar] [CrossRef]
- Li, J.-J.; Shang, X.-Y.; Li, L.-L.; Liu, M.-T.; Zheng, J.-Q.; Jin, Z.L. New cytotoxic azaphilones from Monascus purpureus-fermented rice (red yeast rice). Molecules 2010, 15, 1958–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.-W.; Hsu, L.-C.; Liang, Y.-H.; Kuo, Y.-H.; Pan, T.-M. New bioactive orange pigments with yellow fluorescence from Monascus-fermented Dioscorea. J. Agric. Food Chem. 2011, 59, 4512–4518. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Singh, S.K. Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process Biochem. 2011, 46, 188–192. [Google Scholar] [CrossRef]
- Kaur, B.; Chakraborty, D.; Kaur, H. Production and evaluation of physicochemical properties of red pigment from Monascus purpureus MTCC 410. Internet J. Microbiol. 2008, 7, 1–6. [Google Scholar]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Soumya, K.; Narasimha Murthy, K.; Sreelatha, G.L.; Tirumale, S. Characterization of a red pigment from Fusarium chlamydosporum exhibiting selective cytotoxicity against human breast cancer MCF-7 cell lines. J. Appl. Microbiol. 2018, 125, 148–158. [Google Scholar] [CrossRef]
- Steyn, P.S.; Wessels, P.L.; Marasas, W.F.O. Pigments from Fusarium moniliforme Shledon: Structure and 13C nuclear magnetic resonance assignments of an azaanthraquinone and three naphthoquinones. Tetrahedron 1979, 35, 1551–1555. [Google Scholar] [CrossRef]
- Medenstev, A.G.; Arinbasarova, A.; Akimenko, V.K. Biosynthesis of naphthoquinone pigments by fungi of the genus. Fusarium. Prikl. Biokhim. Mikrobiol. 2005, 41, 573–577. [Google Scholar]
- Pradeep, F.S.; Palaniswamya, M.; Ravib, S.; Thangamanib, A.; Pradeep, B.V. Larvicidal activity of a novel isoquinoline type pigment from Fusarium moniliforme KUMBF1201 against Aedes aegypti and Anopheles stephensi. Process Biochem. 2015, 50, 1479–1486. [Google Scholar] [CrossRef]
- Frandsen, R.J.N.; Rasmussen, S.A.; Knudsen, P.B.; Uhlig, S.; Petersen, D.; Lysoe, E.; Gotfredsen, C.H.; Giese, H.; Larsen, T.O. Black perithecial pigmentation in Fusarium species is due to the accumulation of 5-deoxybostrycoidin-based melanin. Sci. Rep. 2016, 6, 26206. [Google Scholar] [CrossRef] [Green Version]
- Lebeau, J.; Petit, T.; Clerc, P.; Dufossé, L.; Caro, Y. Isolation of two novel purple naphthoquinone pigments concomitant with the bioactive red bikaverin and derivates thereof produced by Fusarium oxysporum. Biotechnol. Prog. 2019, 35, e2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Cai, Y.; Zhou, L.; Huang, P.; Ren, X.; Zuo, A.; Meng, X.; Xu, M.; Liao, X. Benzoquinone from Fusarium pigment inhibits the proliferation of estrogen receptor-positive MCF-7 cells through the NF–κB pathway via estrogen receptor signalling. Int. J. Mol. Med. 2017, 39, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trisuwan, K.; Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Anthraquinone, cyclopentanone, and naphthoquinone derivatives from the sea fan-derived fungi Fusarium spp. PSU–F14 and PSU–F135. J. Nat. Prod. 2010, 73, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Sibero, M.T.; Triningsih, D.W.; Radjasa, O.K.; Sabdono, A.; Trianto, A. Evaluation of antimicrobial activity and identification of yellow pigmented marine sponge-associated fungi from Teluk Awur, Jepara, Central Java. Indones. J. Biotechnol. 2016, 21, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Heo, Y.M.; Kim, K.; Kwon, S.L.; Na, J.; Lee, H.; Jang, S.; Kim, C.H.; Jung, J.; Kim, J.J. Investigation of filamentous fungi producing safe, functional water-soluble pigments. Mycobiology 2018, 46, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Lebeau, J.; Venkatachalam, M.; Fouillaud, M.; Petit, T.; Vinale, F.; Dufossé, L.; Caro, Y. Production and new extraction method of polyketide red pigments produced by ascomycetous fungi from terrestrial and marine habitats. J. Fungi 2017, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, P.; Lee, Y.H.; Nanthakumar, K.; Kamala-Kannan, S.; Dufossé, L.; Mapari, S.A.S.; Oh, B.T. Water-soluble red pigments from Isaria farinosa and structural characterization of the main colored component. J. Basic Microbiol. 2010, 50, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Wat, C.-K.; Mcinnes, A.G.; Smith, D.G.; Wright, J.L.C.; Vining, L.C. The yellow pigments of Beauveria species. Structures of tenellin and bassianin. Can. J. Chem. 1977, 55, 4090–4098. [Google Scholar] [CrossRef] [Green Version]
- Isaka, M.; Chinthanom, P.; Supothina, S.; Tobwor, P. Pyridone and tetramic acid alkaloids from the spider pathogenic fungus Torrubiella sp. BCC 2165. J. Nat. Prod. 2010, 73, 2057–2060. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, H.A.K.; Ramachandran, G.; Subramanian, C.; Karuppan, P. Growth and mass spectrometry profile of Alternaria alternata pigment grown in maize grain extract. J. Microbiol. Biotechnol. Food Sci. 2014, 4, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Suemitsu, R.; Nakamura, A.; Isono, F.; Sano, T. Isolation and identification of Dactylariol from the culture liquid of Alternaria porri (Ellis) Ciferri. Agric. Biol. Chem. 1982, 46, 1693–1694. [Google Scholar] [CrossRef]
- Okuno, T.; Natsume, I.; Sawai, K.; Sawamura, K.; Furusaki, A.; Matsumoto, T. Structure of antifungal and phytotoxic pigments produced by Alternaria sps. Tetrahedron Lett. 1983, 24, 5653–5656. [Google Scholar] [CrossRef]
- Huang, C.H.; Pan, J.H.; Chen, B.; Yu, M.; Huang, H.B.; Zhu, X.; Lu, Y.J.; She, Z.G.; Lin, Y.C. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9–6B from the South China Sea. Mar. Drugs 2011, 9, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.R.; Wakamatsu, K.; Ito, S. Melanin biosynthesis in Cryptococcus neoformans. J. Bacteriol. 1998, 180, 1570–1572. [Google Scholar]
- Harki, E.; Talou, T.; Dargent, R. Purification, characterization and analysis of melanin extracted from Tuber melanosporum vitt. Food Chem. 1997, 58, 69–73. [Google Scholar] [CrossRef]
- Wang, W.; Liao, Y.; Chen, R.; Hou, Y.; Ke, W.; Zhang, B.; Gao, M.; Shao, Z.; Chen, J.; Li, F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea-derived fungus Chaetomium sp. NA–S01–R1. Mar. Drugs 2018, 16, 61. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayanan, T.S.; Ravishankar, J.P.; Venkatesan, G.; Murali, T.S. Characterization of the melanin pigment of a cosmopolitan fungal endophyte. Mycol. Res. 2004, 108, 974–978. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Hanson, J.R.; Yeoh, B.L. The structure and biosynthesis of hinnuliquinone, a pigment from Nodulisporium hinnuleum. J. Chem. Soc. Perkin Trans. 1 1984, 1, 567–569. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.Y.; Song, H.C.; Shen, K.Z.; Wang, L.M.; Sun, R.; Wang, C.R.; Gao, Y.X.; Li, G.H.; Li, L.; et al. Three new naphthoquinone pigments isolated from the freshwater fungus, Astrosphaeriella papuana. Planta Med. 2009, 75, 1339–1343. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; de Menezes, G.C.A.; e Silva, T.R.; Bicas, J.L.; Oliveira, V.M.; Rosa, L.H. Antarctic fungi as producers of pigments. In Fungi of Antarctica; Rosa, L., Ed.; Springer: Cham, Switzerland, 2019; pp. 305–318. [Google Scholar]
- Singh, S.M.; Singh, P.N.; Singh, S.K.; Sharma, P.K. Pigment, fatty acid and extracellular enzyme analysis of a fungal strain Thelebolus microsporus from Larsemann Hills, Antarctica. Polar Rec. 2014, 50, 31–36. [Google Scholar] [CrossRef]
- Fang, L.Z.; Qing, C.; Shao, H.J.; Yang, Y.D.; Dong, Z.J.; Wang, F.; Zhao, W.; Yang, W.Q.; Liu, J.K. Hypocrellin D, a cytotoxic fungal pigment from fruiting bodies of the ascomycete Shiraia bambusicola. J. Antibiot. 2006, 59, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ding, Y.; Tao, G.; Liao, X. Production of 1, 5-dihydroxy-3-methoxy-7-methylanthracene-9, 10-dione by submerged culture of Shiraia bambusicola. J. Microbiol. Biotechnol. 2008, 18, 322–327. [Google Scholar] [PubMed]
- Avalos, J.; Prado-Cabrero, A.; Estrada, A.F. Neurosporaxanthin production by Neurospora and Fusarium. In Microbial Carotenoids from Fungi: Methods and Protocols; Barredo, J.-L., Ed.; Springer Protocols: Totowa, NJ, USA, 2012; pp. 263–274. [Google Scholar]
- Teixeira, M.F.S.; Martins, M.S.; Da Silva, J.C.; Kirsch, L.S.; Fernandes, O.C.C.; Carneiro, A.L.B.; De Conti, R.; Durrn, N. Amazonian biodiversity: Pigments from Aspergillus and Penicillium-characterizations, antibacterial activities and their toxicities. Curr. Trends Biotechnol. Pharm. 2012, 6, 300–311. [Google Scholar]
- Viggiano, A.; Salo, O.; Ali, H.; Szymanski, W.; Lankhorst, P.P.; Nygard, Y.; Bovenberg, R.A.L.; Driessena, A.J.M. Pathway for the biosynthesis of the pigment Chrysogine by Penicillium chrysogenum. Appl. Environ. Microbiol. 2018, 84, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Hosoe, T.; Nozawa, K.; Kawai, K.; Yaguchi, T.; Udagawa, S. Antifungal substances against pathogenic fungi, Talaroconvolutins, from Talaromyces convolutes. J. Nat. Prod. 2000, 63, 768–772. [Google Scholar] [CrossRef]
- Santos, P.O.; Ferraz, C.G.; Soares, A.C.F.; Miranda, F.M.; da Silva, F.; de Abreu Roque, M.R. Sclerotiorin, a novel pigment from Penicillium mallochii. In Proceedings of the 6th Brazilian Conference on Natural Products, Federal University of Espirito Santo Victoria, Vitoria, Brazil, 5–8 November 2017. [Google Scholar]
- Ogihara, J.; Kato, J.; Oishi, K.; Fujimoto, Y. PP-R, 7-(2-Hydroxyethyl)-Monascorubramine, a red pigment produced in the mycelia of Penicillium sp. AZ. J. Biosci. Bioeng. 2001, 91, 44–47. [Google Scholar] [CrossRef]
- Pandey, N.; Jain, R.; Pandey, A.; Tamta, S. Optimisation and characterization of the orange pigment produced by a cold-adapted strain of Penicillium sp. (GBPI_P155) isolated from mountain ecosystem. Mycology 2018, 9, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Dhale, M.A.; Vijay-Raj, A.S. Pigment and amylase production in Penicillium sp. NIOM-02 and its radical scavenging activity. Int. J. Food Sci. Technol. 2009, 44, 2424–2430. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, H.B.; Chen, F.; Hyde, K.D. Production potential of water-soluble Monascus red pigment by a newly isolated Penicillium sp. J. Agric. Technol. 2005, 1, 113–126. [Google Scholar]
- Chintapenta, L.K.; Rath, C.C.; Maringinti, B.; Ozbay, G. Pigment production from a mangrove Penicillium. Afr. J. Biotechnol. 2014, 13, 2668–2674. [Google Scholar]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, S.; Shukla, A.; Mukherjee, S.; Sharma, S.; Guptasarma, P.; Chakraborti, A.K.; Chakrabarti, A. Putative structure and characteristics of red water-soluble pigment secreted by Penicillium marneffei. Med. Mycol. 2007, 45, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez-Zavala, A.; Contreras-Esquivel, J.C.; Lara-Victoriano, F.; Rodriguez-Herrera, R.; Aguilar, C.N. Fungal production of the red pigment using a xerophilic strain Penicillium purpurogenum GH-2. Rev. Mex. Ing. Quim. 2007, 6, 267–273. [Google Scholar]
- Arai, T.; Koganei, K.; Umemura, S.; Kojima, R.; Kato, J.; Kasumi, T.; Ogihara, J. Importance of the ammonia assimilation by Penicillium purpurogenum in amino derivative Monascus pigment, PP-V production. AMB Express 2013, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Sethi, B.K.; Parida, P.; Sahoo, S.L.; Dikshit, B.; Pradhan, C.; Sena, S.; Behera, B.C. Extracellular production and characterization of red pigment from Penicillium purpurogenum BKS9. Alger. J. Nat. Prod. 2016, 4, 379–392. [Google Scholar]
- Ogbonna, C.N.; Aoyagi, H.; Ogbonna, J.C. Isolation and identification of Talaromyces purpurogenus and preliminary studies on its pigment production potentials in solid-state cultures. Afr. J. Biotechnol. 2017, 16, 672–682. [Google Scholar]
- Chadni, Z.; Rahaman, M.H.; Jerin, I.; Hoque, K.M.F.; Reza, M.A. Extraction and optimization of red pigment production as secondary metabolites from Talaromyces verruculosus and its potential use in textile industries. Mycology 2017, 8, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Koolen, H.H.F.; Menezes, L.S.; Souza, M.P.; Silva, F.M.A.; Almeida, F.G.O.; de Souza, A.Q.L.; Nepel, A.; Barison, A.; da Silva, F.H.; Evangelistae, D.E.; et al. Talaroxanthone, a novel xanthone dimer from the endophytic fungus Talaromyces sp. associated with Duguetia stelechantha (Diels) R. E. Fries. J. Braz. Chem. Soc. 2013, 24, 880–883. [Google Scholar]
- Morales-Oyervides, L.; Oliveira, J.; Sousa-Gallagher, M.; Mendez-Zavala, A.; Montanez, J.C. Assessment of the dyeing properties of the pigments produced by Talaromyces spp. J. Fungi 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhai, M.-M.; Li, J.; Jiang, C.-X.; Shi, Y.P.; Di, D.L.; Crews, P.; Wu, Q.-X. The bioactive secondary metabolites from Talaromyces species. Nat. Prod. Bioprospect. 2016, 6, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Pattenden, G. Synthesis of Asperenone, a new pigment from Aspergillus niger and Aspergillus awamori. Tetrahedron Lett. 1969, 10, 4049–4052. [Google Scholar] [CrossRef]
- Youngchim, S.; Morris-Jones, R.; Hay, R.J.; Hamilton, A.J. Production of melanin by Aspergillus fumigatus. J. Med. Microbiol. 2004, 53, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.W.; Solvo, J.J. Isolation and characterization of sexual spore pigments from Aspergillus nidulans. Appl. Environ. Microbiol. 1994, 60, 979–983. [Google Scholar] [PubMed]
- Wang, C.C.; Chiang, Y.M.; Kuo, P.L.; Chang, J.K.; Hsu, Y.L. Norsolorinic acid from Aspergillus nidulans inhibits the proliferation of human breast adenocarcinoma MCF-7 cells via Fas-mediated pathway. Basic Clin. Pharmacol. Toxicol. 2008, 102, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.C.; Eakin, R.E. Studies on the biosynthesis of Aspergillin by Aspergillus niger. J. Appl. Microbiol. 1975, 30, 909–915. [Google Scholar]
- Zabala, A.O.; Xu, W.; Chooi, Y.-H.; Tang, Y. Discovery and characterization of a silent gene cluster that produces azaphilones from Aspergillus niger ATCC 1015 reveal a hydroxylation-mediated pyran-ring formation. Chem. Biol. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoe, T.; Mori, N.; Kamano, K.; Itabashi, T.; Yaguchi, T.; Kawai, K. A new antifungal yellow pigment from Aspergillus nishimurae. J. Antibiot. 2011, 64, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-L.; Li, X.-M.; Wang, B.-G. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium rubrum: Structural elucidation and DPPH radical scavenging activity. J. Biotechnol. 2009, 19, 675–680. [Google Scholar]
- Akilandeswari, P.; Pradeep, B.V. Aspergillus terreus KMBF1501 a potential pigment producer under submerged fermentation. Int. J. Pharm. Pharm. Sci. 2017, 9, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Assante, G.; Camarda, L.; Locci, R.; Merlini, L. Isolation and structure of red pigments from Aspergillus flavus and related species, grown on a differential medium. J. Agric. Food Chem. 1981, 29, 785–787. [Google Scholar] [CrossRef]
- Narendrababu, B.N.; Shishupala, S. Spectrophotometric detection of pigments from Aspergillus and Penicillium isolates. J. Appl. Biol. Biotechnol. 2017, 5, 53–58. [Google Scholar] [CrossRef]
- Cambaza, E. Comprehensive description of Fusarium graminearum pigments and related compounds. Foods 2018, 7, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollenberg, R.D.; Saei, W.; Westphal, K.R.; Klitgaard, C.S.; Nielsen, K.L.; Lysøe, E.; Gardiner, D.M.; Wimmer, R.; Sondergaard, T.S.; Sørensen, J.L. Chrysogine biosynthesis is mediated by a two-module nonribosomal peptide synthetase. J. Nat. Prod. 2017, 80, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Sardaryan, E. Strain of the Microorganism Penicillium oxalicum var. Armeniaca and Its. Application. Patent EP1070136B1, 4 August 2004. [Google Scholar]
- Gupta, C.; Sharma, D.; Aggarwal, S.; Nagpal, N. Pigment production from Trichoderma spp. for dyeing of silk and wool. Int. J. Sci. Nat. 2013, 4, 351–355. [Google Scholar]
- Takahashi, S.; Uchida, K.; Kakinuma, N.; Hashimoto, R.; Yanagisawa, T.; Nakagawa, A. The structures of Pyridovericin and Pyridomacrolidin, new metabolites from the entomopathogenic fungus, Beauveria bassiana. J. Antibiot. 1998, 51, 1051–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.J.; Park, J.P.; Hwang, H.J.; Kim, S.W.; Choi, J.W.; Yun, J.W. Production of red pigment by submerged culture of Paecilomyces sinclairii. Lett. Appl. Microbiol. 2002, 35, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “new” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef] [Green Version]
- Pollmann, H.; Breitenbach, J.; Wolff, H.; Bode, H.B.; Sandmann, G. Combinatorial biosynthesis of novel multi-hydroxy carotenoids in the red yeast Xanthophyllomyces dendrorhous. J. Fungi 2017, 3, 9. [Google Scholar] [CrossRef]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and derivatives from marine-derived fungi: Structural diversity and selected biological activities. Mar. Drugs 2016, 14, 64. [Google Scholar] [CrossRef] [Green Version]
- Fouillaud, M.; Venkatachalam, M.; Llorente, M.; Magalon, H.; Cuet, P.; Dufossé, L. Biodiversity of pigmented fungi isolated from the marine environment in La Reunion Island, Indian Ocean: New resources for colored metabolites. J. Fungi 2017, 3, 36. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Zelena, M.; Cacciola, F.; Ceslova, L.; Girard-Valenciennes, E.; Clerc, P.; Dugo, P.; Mondello, L.; Fouillaud, M.; Rotondo, A.; et al. Partial characterization of the pigments produced by the marine-derived fungus Talaromyces albobiverticillius 30548. Towards a new fungal red colorant for the food industry. J. Food Compost. Anal. 2018, 67, 38–47. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Chen, M.T.; Lin, C.F. Growth, pigment production and protease activity of Monascus purpureus as affected by salt, sodium nitrite, polyphosphate, and various sugars. J. Appl. Microbiol. 2000, 88, 3–37. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Maity, S.; Chattopadhyay, P.; Sarkar, A.; Laskar, S.; Sen, S.K. Characterization of red pigment from Monascus in submerged cultured pigment from Monascus purpureus. J. Appl. Sci. Res. 2009, 5, 2102–2108. [Google Scholar]
- Carvalho, J.C.; Oishi, B.O.; Woiciechowski, A.L.; Pandey, A.; Babitha, S.; Soccol, C.R. Effect of substrates on the production of Monascus biopigments by solid-state fermentation and pigment extraction using different solvents. Indian J. Biotechnol. 2007, 6, 194–199. [Google Scholar]
- Patil, S.A.; Sivanandhan, G.; Thakare, D.B. Effect of physical and chemical parameters on the production of red exopigment from Penicillium purpurogenum isolated from spoilt onion and study of its antimicrobial activity. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 599–609. [Google Scholar]
- Lopes, F.C.; Tichota, D.M.; Pereira, J.Q.; Segalin, J.; Rios, A.D.O. Pigment production by filamentous fungi on agro-industrial byproducts: An eco-friendly alternative. Appl. Biochem. Biotechnol. 2013, 171, 616–625. [Google Scholar] [CrossRef]
- Srianta, I.; Zubaidah, E.; Estiasih, T.; Yamada, M. Comparison of Monascus purpureus growth, pigment production and composition on different cereal substrates with solid state fermentation. Biocatal. Agric. Biotechnol. 2016, 7, 181–186. [Google Scholar] [CrossRef]
- Kantifedaki, A.; Kachrimanidou, V.; Mallouchos, A.; Papanikolaou, S.; Koutinas, A.A. Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Clean. Prod. 2018, 185, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.P.V.; Vendruscolo, F. Production of red pigments by Monascus ruber CCT 3802 using lactose as a substrate. Biocatal. Agric. Biotechnol. 2017, 11, 50–55. [Google Scholar] [CrossRef]
- Chen, M.; Johns, M.R. Effect of pH and nitrogen source on pigment production by Monascus purpureus. Appl. Microbiol. Biotechnol. 1993, 40, 132–138. [Google Scholar] [CrossRef]
- Blanc, P.J.; Loret, M.O.; Santerre, A.L.; Pareilleux, A.; Prome, D.; Prome, J.C.; Laussac, J.P.; Goma, G. Pigments of Monascus. J. Food Sci. 1994, 59, 862–865. [Google Scholar] [CrossRef]
- Pastrana, L.; Blanc, P.J.; Santerre, A.L.; Loret, M.O.; Goma, G. Production of red pigments by Monascus ruber in synthetic media with a strictly controlled nitrogen source. Process Biochem. 1995, 30, 333–341. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Chen, M.; Wang, C. Effect of nitrogen sources on production and photostability of Monascus pigments in liquid fermentation. IERI Procedia 2013, 5, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Z.; Xiong, X.; Liu, Y.; Zhang, J.; Wang, S.; Li, L.; Gao, M. NaCl inhibits citrinin and stimulates Monascus pigments and monacolin K production. Toxins 2019, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Stange, S.; Steudler, S.; Delenk, H.; Werner, A.; Walther, T.; Wagenführ, A. Influence of the nutrients on the biomass and pigment production of Chlorociboria aeruginascens. J. Fumgi 2019, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Carels, M.; Shepherd, D. The effect of pH and amino acids on conidiation and pigment production of Monascus major ATCC 16362 and Monascus rubiginosus ATCC 16367 in submerged shaken culture. Can. J. Microbiol. 1978, 24, 1346–1357. [Google Scholar] [CrossRef]
- Jung, H.; Kim, C.; Kim, K.; Shin, C.S. Color characteristics of Monascus pigments derived by fermentation with various amino acids. J. Agric. Food Chem. 2003, 51, 1302–1306. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Gao, M.; Ding, B.; Zhang, J.; Zhou, Y.; Liu, Y.; Yang, H.; Wu, Q.; Chen, F. Acidic conditions induce the accumulation of orange Monascus pigments during liquid-state fermentation of Monascus ruber M7. Appl. Microbio. Biotech. 2019, 103, 8393–8402. [Google Scholar] [CrossRef]
- Mawthols, K.R.; Deshpande, R.; Ware, D.; Mahajan, M. Effect of pH on pigment production of fungi and their toxicity on seed germination. Ecol. Environ. Conserv. 2005, 11, 325–326. [Google Scholar]
- Mendez, A.; Perez, C.; Montanez, J.C.; Martinez, G.; Aguilar, C.N. Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. J. Zhejiang Univ. Sci. B (Biomed. Biotechnol.) 2011, 12, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Afsharia, M.; Shahidia, F.; Mortazavia, S.A.; Tabatabaia, F.; Es’haghib, Z. Investigating the influence of pH, temperature and agitation speed on yellow pigment production by Penicillium aculeatum ATCC 10409. Nat. Prod. Res. 2015, 29, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Stange, S.; Steudler, S.; Delenk, H.; Werner, A.; Walther, T.; Wagenführ, A. Influence of environmental growth factors on the biomass and pigment production of Chlorociboria aeruginascens. J. Fungi 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Tan, H.; Chen, G.; Wang, L.; Wu, Z. Rising temperature stimulates the biosynthesis of water-soluble fluorescent yellow pigments and gene expression in Monascus ruber CGMCC10910. AMB Express 2017, 7, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Tian, Y.; Zhong, H. Application of a two-stage agitation speed control strategy to enhance yellow pigments production by Monascus anka mutant. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1260–1264. [Google Scholar] [CrossRef]
- Velmurugan, P.; Lee, Y.H.; Venil, C.K.; Lakshmanaperumalsamy, P.; Chae, J.C.; Oh, B.T. Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J. Biosci. Bioeng. 2010, 109, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Buhler, R.M.M.; Muller, B.L.; Moritz, D.E.; Vendruscolo, F.; Oliveira, D.; Ninow, J.L. Influence of light intensity on growth and pigment production by Monascus ruber in submerged fermentation. Appl. Biochem. Biotechnol. 2015, 176, 1277–1289. [Google Scholar] [CrossRef]
- Haggblom, P.; Unestam, T. Blue light inhibits mycotoxin production and increases total lipids and pigmentation in Alternaria alternata. Appl. Environ. Microbiol. 1979, 38, 1074–1077. [Google Scholar]
- Palacio-Barrera, A.M.; Areiza, D.; Zapata, P.; Atehortúa, L.; Correa, C.; Peñuela-Vásquez, M. Induction of pigment production through media composition, abiotic and biotic factors in two filamentous fungi. Biotechnol. Rep. 2019, 21, e00308. [Google Scholar] [CrossRef]
- Tudor, D.; Robinson, S.C.; Cooper, P.A. The influence of moisture content variation on fungal pigment formation in spalted wood. AMB Express 2012, 2, 69. [Google Scholar] [CrossRef] [Green Version]
- Gunsekaran, S.; Poorniammal, R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr. J. Biotechnol. 2008, 7, 1894–1898. [Google Scholar] [CrossRef] [Green Version]
- Chutia, M.; Ahmed, G.U. Optimization of biomass and pigment production by Penicillium species isolated from virgin forest floor. Biotechnol. 2012, 6, 61–69. [Google Scholar]
- Pradeep, F.S.; Pradeep, B.V. Optimization of pigment and biomass production from Fusarium moniliforme under submerged fermentation conditions. Int. J. Pharm. Pharm. Sci. 2013, 5, 526–535. [Google Scholar]
- Ahmad, M.; Panda, B.P. Optimization of red pigment production by Monascus purpureus MTCC 369 under solid-state fermentation using response surface methodology. Songklanakarin J. Sci. Technol. 2014, 36, 439–444. [Google Scholar]
- Devi, S.; Karuppan, P. Influence of culture condition and pH on growth and production of brown pigment from Alternaria alternata. Int. J. Sci. Res. 2014, 3, 458–461. [Google Scholar]
- Santos-Ebinuma, V.C.; Roberto, I.C.; Teixeira, M.F.S.; Pessoa, J., Jr. Improvement of submerged culture conditions to produce colorants by Penicillium purpurogenum. Braz. J. Microbiol. 2014, 45, 731–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyedin, A.; Yazdian, F.; Hatamian-Zarmi, A.; Rasekh, B.; Mir-derikvand, M. Natural pigment production by Monascus purpureus: Bioreactor yield improvement through statistical analysis. Appl. Food Biotechnol. 2015, 2, 23–30. [Google Scholar]
- Patrovsky, M.; Sinovska, K.; Branska, B.; Patakova, P. Effect of initial pH, different nitrogen sources, and cultivation time on the production of yellow or orange Monascus purpureus pigments and the mycotoxin citrinin. Food Sci. Nutr. 2019, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Serrano, R.; González-Menéndez, V.; Rodríguez, L.; Martín, J.; Tormo, J.R.; Genilloud, O. Co-culturing of fungal strains against Botrytis cinerea as a model for the induction of chemical diversity and therapeutic agents. Front. Microbial. 2017, 8, 649. [Google Scholar] [CrossRef]
- Tan, Z.Q.; Leow, H.Y.; Lee, D.C.W.; Karisnan, K.; Song, A.A.L.; Mai, C.W.; Yap, W.S.; Lim, S.H.E.; Lai, K.S. Co-culture Systems for the production of secondary metabolites: Current and future prospects. Open Biotechnol. J. 2019, 13, 18–26. [Google Scholar] [CrossRef]
- Shin, C.S.; Kim, H.J.; Kim, M.J.; Ju, J.Y. Morphological change and enhanced pigment production of Monascus when cocultured with Saccharomyces cerevisiae or Aspergillus oryzae. Biotechnol. Bioeng. 1998, 59, 576–581. [Google Scholar] [CrossRef]
- Frases, S.; Chaskes, S.; Dadachova, E.; Casadevall, A. Induction by Klebsiella aerogenes of a melanin-like pigment in Cryptococcus neoformans. Appl. Environ. Microbiol. 2006, 72, 1542–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.T. Zone lines. In Encyclopedia of Plant Pathology; Malloy, O.C., Murray, T.D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2001; Volume 2, pp. 1217–1218. [Google Scholar]
- Robinson, S. The fine art of decay. Am. Sci. 2014, 102, 206–213. [Google Scholar] [CrossRef]
- Robinson, S.C. Developing fungal pigments for “painting” vascular plants. Appl. Microbiol. Biotechnol. 2012, 93, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Hinsc, E.; Weber, G.L.; Freitas, S. Method of extraction and resolubilisation of pigments from Chlorociboria aeruginosa and Scytalidium cuboideum, two prolific spalting fungi. Coloration Technol. 2014, 130, 221–225. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Oliveira, J.; Sousa-Gallagher, M.; Mendez-Zavala, A.; Montanez, J.C. Perstraction of intracellular pigments through submerged fermentation of Talaromyces spp. in a surfactant-rich media: A novel approach for enhanced pigment recovery. J. Fungi 2017, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Panesar, P.S.; Gurumayum, S.; Rasane, P.; Kumar, V. Optimization of aqueous extraction of orevactaene and flavanoid pigments produced by Epicoccum nigrum. Pigment Resin Technol. 2019, 48, 301–308. [Google Scholar] [CrossRef]
- Weber, G.L.; Boonloed, A.; Naas, K.M.; Koesdjojo, M.T.; Remcho, V.T.; Robinson, S.C. A method to stimulate production of extracellular pigments from wood-degrading fungi using a water carrier. Curr. Res. Environ. Appl. Mycol. 2016, 6, 218–230. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.; Hohn, T.M.; Leathers, T.D. Genetically modifed strains of Fusarium sporotrichioides for production of lycopene and β–carotene. In Proceedings of the Society of Industrial Microbiology Annual Meeting, San Diego, CA, USA, 29 July 2004; p. 91. [Google Scholar]
- Fu, G.; Xu, Y.; Li, Y.; Tan, W. Construction of a replacement vector to disrupt pksCT gene for the mycotoxin citrinin biosynthesis in Monascus aurantiacus and maintain food red pigment production. Asia Pacif. J. Clin. Nutr. 2007, 16, 137–142. [Google Scholar]
- Jia, X.Q.; Xu, Z.N.; Zhou, L.P.; Sung, C.K. Elimination of the mycotoxin citrinin production in the industrial important strain Monascus purpureus SM001. Metab. Eng. 2010, 12, 1–7. [Google Scholar] [CrossRef]
- Westphal, K.; Wollenberg, R.; Herbst, F.A.; Sørensen, J.; Sondergaard, T.; Wimmer, R. Enhancing the production of the fungal pigment aurofusarin in Fusarium graminearum. Toxins 2018, 10, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Li, Y.; Zhang, R.; Yu, J.; Ma, X.; Chen, M.; Wang, Y. Transcriptional regulation contributes more to Monascus pigments diversity in different strains than to DNA sequence variation. World J. Microbiol. Biotechnol. 2019, 35, 138. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U. Colorimetric characterization for comparative analysis of fungal pigments and natural food colorants. J. Agric. Food Chem. 2006, 54, 7027–7035. [Google Scholar] [CrossRef]
- Simpson, B.K.; Benjakul, S.; Klomklao, S. Chapter 37. Natural Food Pigments. In Food Biochemistry and Food Processing, 2nd ed.; Simpson, B.K., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 704–722. [Google Scholar]
- Fink-Gremmels, J.; Leistner, L. Biologische Wirkungen von Monascus purpureus. Fleischwutsch 1989, 69, 116–122. [Google Scholar]
- Kim, D.; Ku, S. Beneficial effects of Monascus sp. KCCM 10093 pigments and derivatives: A mini review. Molecules 2018, 23, 98. [Google Scholar] [CrossRef] [Green Version]
- Martinkova, L.; Juzlova, P.; Vesely, D. Biological activity of polyketide pigments produced by the fungus Monascus. J. Appl. Bacteriol. 1995, 79, 609–616. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Tosin, I.; Giachini, A.J.; Schmidell, W.; Ninow, J.L. Antimicrobial activity of Monascus pigments produced in submerged fermentation. J. Food Process. Preserv. 2014, 38, 1860–1865. [Google Scholar] [CrossRef]
- Manon Mani, V.; Shanmuga Priya, M.; Dhaylini, S.; Preethi, K. Antioxidant and antimicrobial evaluation of bioactive pigment from Fusarium sp. isolated from the stressed environment. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 1147–1158. [Google Scholar]
- Saravanan, D.; Radhakrishnan, M. Antimicrobial activity of pigments produced by fungi from the Western Ghats. J. Chem. Pharm. Res. 2016, 8, 634–638. [Google Scholar]
- Yolmeh, M.; Hamedi, H.; Khomeiri, M. Antimicrobial activity of pigments extracted from Rhodotorula glutinis against some bacteria and fungi. Zahedan J. Res. Med Sci. 2016, 18, e4954. [Google Scholar] [CrossRef] [Green Version]
- Poorniammal, R.; Parthiban, M.; Gunasekaran, S.; Murugesan, R.; Thilagavathi, G. Natural dye production from Thermomyces sp. fungi for textile application. Indian J. Fibre Text. Res. 2013, 38, 276–279. [Google Scholar]
- Devi, S.; Karuppan, P. Reddish brown pigments from Alternaria alternata for textile dyeing and printing. Indian J. Fibre Text. Res. 2015, 40, 315–319. [Google Scholar]
- Prathiban, M.; Thilagavathi, G.; Viju, S. Development of antibacterial silk sutures using the natural fungal extract for healthcare applications. J. Text. Sci. Eng. 2016, 6, 249. [Google Scholar]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendruscolo, F.; Buhler, R.M.M.; de Carvalho, J.C.; de Oliveira, D.; Moritz, D.E.; Schmidell, W.; Ninow, J.L. Monascus: A reality on the production and application of microbial pigments. J. Appl. Biochem. Biotechnol. 2016, 178, 211–223. [Google Scholar] [CrossRef]
- Li, F.; Xue, F.; Yu, X. GC-MS, FTIR and Raman analysis of antioxidant components of red pigments from Stemphylium lycopersici. Curr. Microbiol. 2017, 74, 532–539. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Sakthi, A.R. Evaluation of in vitro antioxidant activity of fungal pigments. Pharma Innov. J. 2019, 8, 326–330. [Google Scholar]
- Malik, K.; Tokas, J.; Anand, R.C. Characterization and cytotoxicity assay of pigment-producing microbes. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 370–376. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Sakthi, A.R.; Gunasekaran, S. Subacute dermal toxicity of Thermomyces sp. and Penicillium purpurogenum pigments in wistar rats. Int. J. Chem. Stud. 2019, 7, 630–634. [Google Scholar]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Kiyota, A.; Yasukawa, K.; Sakamoto, N.; Kimura, Y.; Suzuki, T.; Takayasu, J.; Nishino, H. Anti-tumor-initiating effects of Monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers. 2005, 2, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Su, N.-W.; Lin, Y.-L.; Lee, M.-H.; Ho, C.-Y. Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J. Agric. Food Chem. 2005, 53, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Hill, H. The function of melanin or six blind people examine an elephant. BioEssays 1992, 14, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sajid, S.; Akber, N. Applications of fungal pigments in biotechnology. Pure Appl. Biol. 2018, 7, 922–930. [Google Scholar] [CrossRef]
- Velmurugan, P.; Kim, M.J.; Park, J.S.; Karthikeyan, K.; Lakshmanaperumalsamy, P.; Lee, K.J.; Park, Y.J.; Oh, B.T. Dyeing of cotton yarn with five water-soluble fungal pigments obtained from five fungi. Fibers Polym. 2010, 11, 598–605. [Google Scholar] [CrossRef]
- Mabrouk, A.M.; El-Kkhrisy, E.A.M.; Youssef, Y.A.; Asem, M.A. Production of textile reddish brown dyes by fungi. Malays. J. Microbiol. 2011, 7, 33–40. [Google Scholar]
- Sharma, D.; Gupta, C.; Aggarwal, S.; Nagpal, N. Pigment extraction from fungus for textile dyeing. Indian J. Fibre Text. Res. 2012, 37, 68–73. [Google Scholar]
- Aishwarya, A.D. Extraction of natural dyes from fungus—An alternate for textile dyeing. J. Nat. Sci. Res. 2014, 4, 1–6. [Google Scholar]
- Weber, G.; Chen, H.L.; Hinsch, E.; Freitas, S.; Robinson, S. Pigments extracted from the wood-staining fungi Chlorociboria aeruginosa, Scytalidium cuboideum, and S. ganodermophthorum show potential for use as textile dyes. Coloration Technol. 2014, 130, 445–452. [Google Scholar] [CrossRef]
- Robinson, S.C.; Tudor, D.; Cooper, P.A. Utilizing pigment-producing fungi to add commercial value to American beech (Fagus grandifolia). Appl. Microbial. Biotechnol. 2012, 93, 1041–1048. [Google Scholar] [CrossRef]
- Giesbers, G.; Van Schenck, J.; Gutierrez, S.V.; Robinson, S.; Ostroverkhova, O. Fungi-derived pigments for sustainable organic (opto) electronics. MRS Adv. 2018, 3, 3459–3464. [Google Scholar] [CrossRef] [Green Version]
- Giesbers, G.; Van Schenck, J.; Quinn, A.; Van Court, R.; Vega Gutierrez, S.M.; Robinson, S.C.; Ostroverkhova, O. Xylindein: Naturally produced fungal compound for sustainable (opto) electronics. ACS Omega 2019, 4, 13309–13318. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Fungal Species | Pigments | References |
|---|---|---|
| Monascus species | ||
| Monascus pilosus | Citrinin (yellow) | [61] |
| Monascus purpureus | Monascin (yellow), monascorubrin (orange), monascorubramine (red), monapurone A–C (yellow), monasphilone A and B (yellow), ankaflavin (yellow), rubropunctamine (purple-red), rubropunctatin (orange), monopilol A–D (yellow), citrinin (yellow), 9–(1–hydroxyhexyl)–3–(2–hydroxypropyl)–6a–methyl–9,9a–dihydrofuro[2,3–h] isoquinoline–6,8(2H,6aH)–dione (red), uncharacterized (red) | [56,57,58,59,60,61] |
| Monascus ruber | Monascin (yellow), monascorubramine (red), monascorubrin (orange), ankaflavin (yellow), citrinin (yellow), rubropunctamine (purple-red), rubropunctatin (orange), N–glucosylrubropunctamine (red), N–glucosylmonascorubramine (red), monarubrin (pale yellow), rubropunctin (pale yellow) | [52,54,61] |
| Monascus species | Ankaflavin (yellow) *, monascorubramine (red) *, rubropunctatin (orange) * | [25] |
| Fusarium species | ||
| Fusarium acuminatum, F. avenaceum, F. tricinctum | Antibiotic Y (yellow), aurofusarin (red) | [61] |
| Fusarium chlamydosporum | Uncharacterized (red) | [62] |
| Fusarium culmorum | Aurofusarin (red), fuscofusarin (yellow), rubrofusarin (red) | [61] |
| Fusarium fujikuroi (formerly known as Fusarium moniliforme/ Fusarium verticillioides) | Bikaverin (red), norbikaverin (red), O–demethylanhydrofusarubin (red), 8–O–methybostrycoidin, 2–(4–((3E,5E)–14–aminotetradeca–3,5–dienyloxy)butyl)–1,2,3,4–tetrahydroisoquinolin–4–ol (ATDBTHIQN) (pink), neurosporaxanthin (orange), β–carotene (red-orange), fusarubin (red), O–demethylfusarubin, O–methyljavanicin, O–methylsolaniol (orange-red) | [43,61,63,64,65] |
| Fusarium graminearum | Aurofusarin (red,) rubrofusarin (red), 5–deoxybostrycoidin anthrone (green), 6–O–demethyl– 5–deoxybostrycoidin anthrone (blue), purpurfusarin (purple), 6–O–demethyl–5–deoxybostrycoidin (yellow), 5–deoxybostrycoidin (red) | [64,66] |
| Fusarium oxysporum | 2,7–dimethoxy–6–(acetoxyethyl)juglone (yellow), bikaverin (red), bostrycoidin (red), nectriafurone (yellow), norjavanicin (red), O–methyl–6– hydroxynorjavanicin (yellow), O–methylanhydrofusarubin (orange-red), O–methylfusarubin (red), O–methyljavanicin, 2–acetyl–3,8–dihydroxy–6–methoxy anthraquinone (yellow), 2–(1–hydroxyethyl)–3,8–dihydroxy–6–methoxy anthraquinone (orange), neurosporaxanthin (orange), β–carotene (red-orange), uncharacterized naphthaquinones (purple) | [43,47,61,64,67] |
| Fusarium poae, F. sambucinum | Aurofusarin (red) | [61] |
| Fusarium solani | Fusarubin (red), O–methyldihydrofusarubin (red), O–ethylfusarubin (red), isomarticins (red) | |
| Fusarium sporotrichioides | Aurofusarin (red), β–carotene (yellow-orange) **, lycopene (red) ** | [25,61] |
| Fusarium stilboides | Antibiotic Y (yellow), aurofusarin (red), nectriafurone (yellow) | [61] |
| Fusarium venenatum | Aurofusarin (red), rubrofusarin (red) | |
| Fusarium sp. | Benzoquinone (yellow) | [68] |
| Fusarium sp. PSU–F14 and PSU–F135 | Fusarnaphthoquinones B (red), fusarnaphthoquinones C (red) | [69] |
| Fusicollaaquaeductuum (Formerly Known as Fusarium aquaeductuum) | ||
| Fusicolla aquaeductuum | Neurosporaxanthin (orange), β–carotene (red-orange) | [43] |
| Albonectria rigidiuscula (Formerly Known as Fusarium decemcellulare) | ||
| Albonectria rigidiuscula | Javanicin (red–orange), fusarubin (red), anhydrojavanicin, anhydrofusarubin, bostricoidin (red), novarubin | [64] |
| Trichoderma species | ||
| Trichoderma harzianum | Pachybasin (yellow), chrysophanol (orange-red), emodin (yellow), 1–hydroxy–3–methyl–anthraquinone, 1,8–dihydroxy–3–methyl–anthraquinone, T22 azaphilone | [25] |
| Trichoderma polysporum | Pachybasin (yellow), chrysophanol (orange-red), emodin (yellow) | |
| Trichoderma viride | Pachybasin (yellow), chrysophanol (orange-red), emodin (yellow), 1,3,6,8–tetrahydroxyanthraquinone, 2,4,5,7– tetrahydroxyanthraquinone | |
| Trichoderma aureoviride | Pachybasin (yellow), chrysophanol (orange-red) | |
| Trichoderma afrharzianum, Trichoderma pyramidale, Trichoderma parareesei (formerly known as Trichoderma atroviride), Trichoderma sp. 1 | Uncharacterized (yellow) | [70,71] |
| Trichoderma parceramosum | Uncharacterized (red) | [72] |
| Cordyceps farinosa (Formerly Known as Isaria farinosa) | ||
| Cordyceps farinosa | Anthraquinone derivative | [73] |
| Ophiocordycepsunilateralis (Formerly Known as Cordyceps unilateralis) | ||
| Ophiocordyceps unilateralis | Erythrostominone (red), 3,5,8–TMON * (red), deoxyerythrostominone (red), deoxyerythrostominol (red), 4–O–methyl erythrostominone (red), epierythrostominol (red), naphthoquinones (deep blood red) ** | [25] |
| Beauveria species | ||
| Beauveria basiana | Tenellin (yellow), bassianin (yellow), pyridovericin (pale yellow), pyridomacrolidin (pale yellow), oosporein (red) | [25,74] |
| Beauveria brongniartii (formerly known as Beauveria tenella) | Tenellin (yellow), bassianin (yellow) | |
| Torrubiella species | ||
| Torrubiella sp. | Torrubiellones A–D (yellow) | [75] |
| Lecanicillium species | ||
| Lecanicillium aphanocladii | Oosporein (red) | [41] |
| Hyperdermium species | ||
| Hyperdermium bertonii | Skyrin (orange-red) | [25] |
| Daldinia species | ||
| Daldinia bambusicol, Daldinia caldariorum, Daldinia childiae, Daldinia clavata, Daldinia fissa, Daldinia grandis, Daldinia lloydi, Daldinia loculata, Daldinia petriniae, Daldinia singularis | BNT (1,1ˊ–Binaphthalene–4,4ˊ–5,5́–tetrol) (yellow), daldinol (dark brown), 8–methoxy–1–napthol, 2–hydroxy–5–methylchromone | [25] |
| Daldinia concentrica | BNT (1,1ˊ–Binaphthalene–4,4ˊ–5,5́–tetrol) (yellow), daldinol, 8–methoxy–1–napthol, 2–hydroxy–5–methylchromone, daldinal A–C (yellow), daldinin A–C (green-olivaceous-isabelline) | |
| Daldinia eschscholzii | BNT (1,1ˊ–Binaphthalene–4,4ˊ–5,5́–tetrol) (yellow), daldiol (dark brown), 8–methoxy–1–napthol, 2–hydroxy–5–methylchromone, daldinal A–C (yellow) | |
| Jackrogersella cohaerens (Formerly Known as Annulohypoxylon cohaerens) | ||
| Jackrogersella cohaerens | Cohaerin A | [25] |
| Hypoxylon species | ||
| Hypoxylon fragiforme | Hypoxyxylerone (green), fragiformins A–B, cytochalasin H (white), mitorubrin azaphilones (red) | [25] |
| Hypoxylon howeanum | Mitorubrin azaphilones (red) | |
| Hypoxylon lechatii | Vermelhotin (orange-red), hypoxyvermelhotins A–C (orange-red) | |
| Hypoxylon fuscum | Daldinin A–C (green-olivaceous-isabelline) | |
| Hypoxylon fulvo–sulphureum | Mitorubrinol derivatives | |
| Hypoxylon sclerophaeum | Hypoxylone (orange) | |
| Hypoxylon rickii | Rickenyl B (red), rickenyl D (brown) | |
| Hypoxylon lenormandii, Hypoxylon jaklitschii | Lenormandins A–G (yellow) | |
| Hypoxylon rubiginosum | Mitorubrin (orange), rubiginosin (orange-brown), hypomiltin (yellowish-green) | |
| Alternaria species | ||
| Alternaria alternata | Alternariol (red), altenuene (red-violet), alternarienoic acid (red), alternariol-5-methyl ether (red-brown), tenuazoic acid (orange-red), alterperylenol (red), stemphyperylenol (yellow–orange-red) | [76] |
| Aternaria dauci | Uncharacterized (red) | [25,61] |
| Aternaria porri | Altersolanol A (yellow-orange), dactylariol | [25,61,77] |
| Aternaria solani, Aternaria tomatophila | Altersolanol A (yellow-orange) | [25,61] |
| Alternaria species | Alterperylenol (red), dihydroalterperylenol (dark purple) | [78] |
| Alternaria sp. ZJ9–6B | Alterporriol K–M (red) | [79] |
| Curvularia species | ||
| Curvularia lunata | Chrysophanol (red), cynodontin (bronze), helminthosporin (maroon), erythroglaucin (red), catenarin (red) | [25,61] |
| Sanghuangporusspecies | ||
| Sanghuangporus baumii | Uncharacterized (yellow) | [71] |
| Clonostachys species | ||
| Clonostachys intermedia | Uncharacterized (yellow) | [71] |
| Pyrenophora species (Previously Known as species of Drechslera) | ||
| Pyrenophora teres, Pyrenophora graminea, Pyrenophora tritici–repentis, Pyrenophora grahamii, Pyrenophora dictyoides, Pyrenophora chaetomioides | Catenarin (red), cynodontin (bronze), helminthosporin (maroon), tritisporin (reddish-brown), erythroglaucin (red) | [25,61] |
| Exophialaspecies | ||
| Exophiala dermatitidis (formerly known as Wangiella dermatitidis) | Melanin (black-brown) | [44] |
| Sporothrixspecies | ||
| Sporothrix schenckii | Melanin (black-brown) | [44] |
| Cryptococcus species | ||
| Cryptococcus neoformans | Dihydroxy phenyl alanine-melanin | [29,80] |
| Tuberspecies | ||
| Tuber melanosporum | Melanin (black) | [29,81] |
| Polyporus species | ||
| Lentinus brumalis (formerly known as Polyporus brumalis) | Melanin (black) | [34,35] |
| Cerioporus squamosus (formerly known as Polyporus squamosus) | Melanin (black) | |
| Xylaria species | ||
| Xylaria polymorpha | Melanin (black) | [34,35] |
| Fomesspecies | ||
| Fomes fomentarius | Melanin (black) | [34,35] |
| Oxyporusspecies | ||
| Oxyporus populinus | Melanin (black) | [34] |
| Trametes species | ||
| Trametes versicolor | Melanin (black) | [34,35] |
| Inonotus species | ||
| Inonotus hispidus | Melanin (black), uncharacterized (yellow) | [34,35,36] |
| Chlorociboria species | ||
| Chlorociboria aeruginascens | Xylindein (green), xylindein quinol (yellow) | [33] |
| Chlorociboria aeruginosa | Xylindein (green) | [37,39] |
| Scytalidium species | ||
| Scytalidium cuboideum | Draconin red (red) | [37,39] |
| Scytalidium ganodermophthorum | Uncharacterized (yellow) | [36,39] |
| Scytalidium lignicola | Uncharacterized (yellow) | [36,39] |
| Epicoccum species | ||
| Epicoccum nigrum | Carotenoids, chromanone (yellow), epicoccarines A–B, epicocconone (fluorescent yellow), epipyridone (red), flavipin (brown), isobenzofuran derivatives (yellow to brown), orevactaene (yellow) | [41,61] |
| Chaetomium species | ||
| Chaetomium cupreum | Oosporein (red), rotiorinols A–C (red), rubrorotiorin (red) | [25] |
| Chaetomium globosum | Chaetoviridins A–D (yellow), chaetoglobin A–B, chaetomugilins A–F, cochliodinol (purple) | |
| Chaetomium sp. NA–S01–R1 | Chaephilone–C (yellow), chaetoviridides A–C (red) | [82] |
| Achaetomium species | ||
| Achaetomium sp. | Parietin (orange) | [25] |
| Phyllosticta species | ||
| Phyllosticta capitalensis | Melanin (black) | [83] |
| Cladosporium species | ||
| Cladosporium cladosporioides | Calphostins A–D and I (red) | [61] |
| Nodulisporium species | ||
| Nodulisporium hinnuleum | Hinnuliquinone (red) | [84] |
| Astrosphaeriella species | ||
| Astrosphaeriella papuana | Astropaquinones A–C (orange) | [85] |
| Arthrobotrys species | ||
| Arthrobotrys ferox | Carotenoid | [86] |
| Thelebolus species | ||
| Thelebolus microsporus | β-carotene (orange) | [86,87] |
| Shiraia species | ||
| Shiraia bambusicola | Shiraiarin (red), hypocrellin D (orange-red) | [88,89] |
| Paecilomyces species | ||
| Paecilomyces sinclairii | Uncharacterized (red) ** | [25,61] |
| Neurospora species | ||
| Neurospora crassa | Neurosporaxanthin (yellow-orange), phytoene (yellow-orange), β–carotene (red-orange), lycopene (red), neurosporen (yellow-orange), spirilloxanthin (violet), ϒ–carotene (yellow-orange), β–carotene (yellow-orange) ** | [25,90] |
| Neurospora sitophila | Neurosporaxanthin (yellow-orange) | [26] |
| Neurospora intermedia | Uncharacterized (yellow-orange), a mixture of carotenoids | |
| Blakesleaspecies | ||
| Blakeslea trispora | β–carotene (yellow-orange) *, lycopene (red) * | [25] |
| Ashbya species | ||
| Ashbya gossypi | Riboflavin (yellow) * | [25] |
| Phycomyces species | ||
| Phycomyces blakesleeanus | β–carotene (yellow-orange) ** | [25] |
| Mucor species | ||
| Mucor circinelloides | β–carotene (yellow-orange) *** | [25] |
| Lactarius species | ||
| Lactarius sp. | Azulenes (blue) ** | [25] |
| Penicillium species | ||
| Penicillium atramentosum | Uncharacterized (dark brown) | [61,91] |
| Penicillium atrosanguineum | Phoenicin (red), uncharacterized (yellow and red) | |
| Penicillium atrovenetum | Atrovenetin (yellow), norherqueinone (red) | |
| Penicillium aurantiogriseum | Uncharacterized | |
| Penicillium brevicompactum, Penicillium simplicissimum | Xanthoepocin (yellow) | |
| Penicillium chrysogenum | Sorbicillins (yellow), xanthocillin (yellow), chrysogine (yellow) | [61,92] |
| Penicillium citrinum | Anthraquinones (yellow), citrinin (yellow) | [61] |
| Penicillium convolutum (formerly known as Talaromyces convolutus) | Talaroconvolutins A–D, ZG–1494α | [93] |
| Penicillium cyclopium | Viomellein (reddish–brown), xanthomegnin (orange) | [61] |
| Penicillium discolor | Uncharacterized | |
| Penicillium echinulatum | Uncharacterized (yellow) | |
| Penicillium flavigenum | Xanthocillin (yellow), dihydrotrichodimerol (yellow) | [41,61] |
| Penicillium freii, Penicillium viridicatum | Viomellein (reddish-brown), vioxanthin, xanthomegnin (orange) | [61] |
| Penicillium herquei | Atrovenetin (yellow), herqueinones (red and yellow) | |
| Penicillium melinii | Atrovenetin (yellow) | [91] |
| Penicillium miczynskii | Uncharacterized (red) | [71] |
| Penicillium mallochii | Sclerotiorin (yellow) | [94] |
| Penicillium oxalicum | Arpink red™, anthraquinone derivative (red), secalonic acid D (yellow), anthraquinones (red and other hues) * | [25,61] |
| Penicillium paneum | Uncharacterized (red) | [61] |
| Penicillium persicinum | Uncharacterized (cherry red) | |
| Penicillium sp. AZ | PP–V (violet), PP–R (red) | [95] |
| Penicillium sp. (GBPI_P155) | Uncharacterized (orange) | [96] |
| Penicillium sp. NIOM–02 | Uncharacterized (red) | [97] |
| Penicillium sp. | Uncharacterized (red) | [98,99] |
| Talaromyces species | ||
| Talaromyces aculeatus (formerly known as Penicillium aculeatum) | Uncharacterized | [61] |
| Talaromyces atroroseus | Mitorubrin (red), monascorubrin (red), PP–R (red), glauconic acid (red), purpuride (red), ZG–1494α (red), azaphilones (red) *** | [25,100] |
| Talaromyces albobiverticillius, Talaromyces amestolkiae, Talaromyces stollii | Monascus–like azaphilones (red) | [25] |
| Talaromyces cnidii, Talaromyces coalescens | Monascus–like azaphilones (red), uncharacterized (red) | |
| Talaromyces funiculosus (formerly known as Penicillium funiculosum) | Ankaflavain (yellow), uncharacterized | [61] |
| Talaromyces islandicus (formerly known as Penicillium islandicum) | Emodin (yellow), skyrin (orange), erythroskyrin (orange-red), luteoskyrin (yellow) | |
| Talaromyces marneffei (formerly known as Penicillium marneffiei) | Monascorubramine (purple-red), mitorubrinol (orange-red), rubropunctatin (orange), purpactin, herqueinone like (brick red), secalonic acid D (yellow) | [61,101] |
| Talaromyces pinophilus (formerly known as Penicillium pinophilum) | Azaphilones, uncharacterized | [25,61] |
| Talaromyces purpureogenus (formerly known as Penicillium purpureogenum) | Mitorubrin (yellow), mitorubrinol (orange-red), PP–R (purple-red), purpurogenone (yellow-orange), rubropunctatin (red), N–glutarylmonascorubramine, N–glutarylrubropunctamine, uncharacterized (red), azaphilones (red) *** | [25,61,102,103,104,105] |
| Talaromyces ruber (formerly known as Penicillium crateriforme) | Uncharacterized, Monascus–like azaphilones | [25] |
| Talaromyces rugulosus (formerly known as Penicillium rugulosum) | Rugulosin (yellow) | [61] |
| Talaromyces variabillis (formerly known as Penicillium variabile) | Rugulosin (yellow) | [61] |
| Talaromyces vericulosus | Uncharacterized (red) | [106] |
| Talaromyces sp. DgCr22.1b | Talaroxanthone (yellow) | [107] |
| Talaromyces siamensis, Talaromyces sp. | Uncharacterized (red) | [71,108] |
| Talaromyces sp. | N–threonine rubropunctamine (red) | [72] |
| Hamigera avellanea (Formerly Known as Talaromyces avellaneus) | ||
| Hamigera avellanea | Emodin (yellow), erythroglaucin (red), catenarin (red) | [109] |
| Aspergillus species | ||
| Aspergillus amstelodami | Physcion (yellow), erythroglaucin (red), flavoglaucin (yellow), auroglaucin (orange-red) | [25] |
| Aspergillus awamori | Asperenone (yellow) | [110] |
| Aspergillus chevalieri | Physcion (yellow), erythroglaucin (red), flavoglaucin (yellow), auroglaucin (orange-red), catenarin (red), rubrocristin (red) | [25] |
| Aspergillus cristatus | Emodin (yellow), questin (yellow to orange-brown), erythroglaucin (red), physcion (yellow), catenarin (red), rubrocristin (red) | [25,61] |
| Aspergillus echinulatum, Aspergillus glaber, Aspergillus spiculosus, Aspergillus umbrosus | Erythroglaucin (red), physcion (yellow), catenarin (red), rubrocristin (red) | [25] |
| Aspergillus fumigatus | Melanin (dark brown-black) | [25,111] |
| Aspergillus falconensis, Aspergillus fruticulosus | Falconensins A–H (yellow), falconensones A1 and B2 (yellow), zeorin (yellow) | [25] |
| Aspergillus glaucus | Physcion (yellow), emodin (yellow), questin (yellow to orange-brown), erythroglaucin (red), catenarin (red), rubrocristin (red), flavoglaucin (yellow), auroglaucin (orange-red), aspergin (yellow) | [25,61] |
| Aspergillus intermedius, Aspergillus leucocarpus, Aspergillus tonophilus | Physcion (yellow), erythroglaucin (red) | |
| Aspergillus ochraceus | Viomellein (reddish-brown), vioxanthin, xanthomegnin (orange) | |
| Aspergillus melleus, Aspergillus sulphureus, Aspergillus westerdijkiae | Viomellein (reddish-brown), rubrosulphin (red), viopurpurin (purple), xanthomegnin (orange) | |
| Aspergillus nidulans | Ascoquinone A (red), norsolorinic acid, sterigmatocystin (yellow), melanin (dark brown-black) | [25,112,113] |
| Aspergillus niger | Flavioline (orange-red), N-naptho–γ–pyrones (yellow), aspergillin (black), azanigerones A–F, asperenone (yellow), melanin (dark brown-black) | [25,61,110,114,115] |
| Aspergillus nishimurae | Anishidiol (yellow) | [116] |
| Aspergillus parvathecia, Aspergillus rugulosus, Aspergillus versicolor | Sterigmatocystin (yellow) | [25] |
| Aspergillus purpureus | Epurpurins A–C (yellow) | |
| Aspergillus repens | Emodin (yellow), physcion (yellow), erythroglaucin (red), catenarin (red), rubrocristin (red), questin (yellow to orange-brown) | |
| Aspergillus ruber | Catenarin (red), rubrocristin (red), emodin (orange), asperflavin (yellow), eurorubrin (Brown), questin (yellow to orange-brown), 3–O–(α–D–ribofuranosyl)–questin (orange), 2–O–methyl–9–dehydroxyeurotinone, 2–O–methyl–4–O–(α–D–ribofuranosyl)–9–dehydroxyeurotinone, 2–O–methyleurotinone | [25,117] |
| Aspergillus sclerotioniger | Uncharacterized (yellow) | [61] |
| Aspergillus sclerotiorum | Neoaspergillic acid (yellow-green) | [91] |
| Aspergillus terreus | Uncharacterized (yellow) | [118] |
| Aspergillus sp. | Ferriaspergillin (red), ferrineoaspergillin (red) | [119] |
| Aspergillus sp. | Uncharacterized (yellow) | [120] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal Pigments and Their Prospects in Different Industries. Microorganisms 2019, 7, 604. https://doi.org/10.3390/microorganisms7120604
Lagashetti AC, Dufossé L, Singh SK, Singh PN. Fungal Pigments and Their Prospects in Different Industries. Microorganisms. 2019; 7(12):604. https://doi.org/10.3390/microorganisms7120604
Chicago/Turabian StyleLagashetti, Ajay C., Laurent Dufossé, Sanjay K. Singh, and Paras N. Singh. 2019. "Fungal Pigments and Their Prospects in Different Industries" Microorganisms 7, no. 12: 604. https://doi.org/10.3390/microorganisms7120604
APA StyleLagashetti, A. C., Dufossé, L., Singh, S. K., & Singh, P. N. (2019). Fungal Pigments and Their Prospects in Different Industries. Microorganisms, 7(12), 604. https://doi.org/10.3390/microorganisms7120604