Using Oxidative Electrodes to Enrich Novel Members in the Desulfobulbaceae Family from Intertidal Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioelectrochemical Reactor Configuration and Operation
2.2. Microbial Community Characterizations and Analyses
2.3. Microscopic Examinations
3. Results
3.1. The Current and Voltage of the Bioelectrochemical Reactors
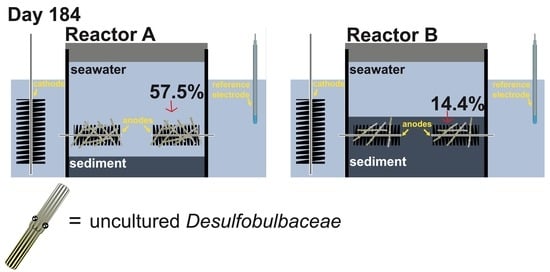
3.2. Microbial Communities in Bioelectrochemical Reactors
3.3. Microscopic Examination of the Electrodes and Biomass inside of the Bioelectrochemical Reactors
4. Discussion
4.1. Enriching Novel Desulfobulbaceae by Using the Bioelectrochemical Reactors
4.2. Desulfobulbaceae Observed inside the Bioelectrochemical Reactors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Lesnik, K.L.; Liu, H. Stay Connected: Electrical Conductivity of Microbial Aggregates. Biotechnol. Adv. 2017, 35, 669–680. [Google Scholar] [CrossRef]
- Lovley, D.R. Happy Together: Microbial Communities That Hook up to Swap Electrons. ISME J. 2016, 11, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jangir, Y.; French, S.; Momper, L.M.; Moser, D.P.; Amend, J.P.; El-Naggar, M.Y. Isolation and Characterization of Electrochemically Active Subsurface Delftia and Azonexus Species. Front. Microbiol. 2016, 7, 756. [Google Scholar] [CrossRef] [Green Version]
- McGlynn, S.E.; Chadwick, G.L.; Kempes, C.P.; Orphan, V.J. Single Cell Activity Reveals Direct Electron Transfer in Methanotrophic Consortia. Nature 2015, 526, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intercellular Wiring Enables Electron Transfer between Methanotrophic Archaea and Bacteria. Nature 2015, 526, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Kuever, J. The Family Desulfobulbaceae. In The Prokaryotes: Deltaproteobacteria and Epsilonproteobacteria; Rosenberg, E., DeLong Edward, F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 75–86. ISBN 978-3-642-39044-9. [Google Scholar]
- Lovley, D.R.; Phillips, E.J.P. Novel Processes for Anaerobic Sulfate Production from Elemental Sulfur by Sulfate-Reducing Bacteria. Appl. Environ. Microb. 1994, 60, 2394–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannenberg, S.; Kroder, M.; Dilling, W.; Cypionka, H. Oxidation of H2, Organic Compounds and Inorganic Sulfur Compounds Coupled to Reduction of O2 or Nitrate by Sulfate-Reducing Bacteria. Arch. Microbiol. 1992, 158, 93–99. [Google Scholar] [CrossRef]
- Dyksma, S.; Lenk, S.; Sawicka, J.E.; Mußmann, M. Uncultured Gammaproteobacteria and Desulfobacteraceae Account for Major Acetate Assimilation in a Coastal Marine Sediment. Front. Microbiol. 2018, 9, 3124. [Google Scholar] [CrossRef] [PubMed]
- Vliet, D.M.; Meijenfeldt, F.A.B.; Dutilh, B.E.; Villanueva, L.; Damsté, J.S.S.; Stams, A.J.M.; Sánchez-Andrea, I. The Bacterial Sulfur Cycle in Expanding Dysoxic and Euxinic Marine Waters. Environ. Microbiol. 2020, 23, 2834–2857. [Google Scholar] [CrossRef]
- Kouzuma, A.; Ishii, S.; Watanabe, K. Metagenomic Insights into the Ecology and Physiology of Microbes in Bioelectrochemical Systems. Bioresour. Technol. 2018, 255, 302–307. [Google Scholar] [CrossRef]
- Holmes, D.E.; Bond, D.R.; Lovley, D.R. Electron Transfer by Desulfobulbus Propionicus to Fe(III) and Graphite Electrodes. Appl. Environ. Microb. 2004, 70, 1234–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, D.E.; Bond, D.R.; O’Neil, R.A.; Reimers, C.E.; Tender, L.R.; Lovley, D.R. Microbial Communities Associated with Electrodes Harvesting Electricity from a Variety of Aquatic Sediments. Microb. Ecol. 2004, 48, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Malkin, S.Y.; Rao, A.M.; Seitaj, D.; Vasquez-Cardenas, D.; Zetsche, E.-M.; Hidalgo-Martinez, S.; Boschker, H.T.; Meysman, F.J. Natural Occurrence of Microbial Sulphur Oxidation by Long-Range Electron Transport in the Seafloor. ISME J. 2014, 8, 1843–1854. [Google Scholar] [CrossRef]
- Trojan, D.; Schreiber, L.; Bjerg, J.T.; Bggild, A.; Yang, T.; Kjeldsen, K.U.; Schramm, A. A Taxonomic Framework for Cable Bacteria and Proposal of the Candidate Genera Electrothrix and Electronema. Syst. Appl. Microbiol. 2016, 39, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, C.; Larsen, S.; Song, J.; Dong, M.; Besenbacher, F.; Meyer, R.L.; Kjeldsen, K.U.; Schreiber, L.; Gorby, Y.A.; El-Naggar, M.Y.; et al. Filamentous Bacteria Transport Electrons over Centimetre Distances. Nature 2012, 491, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Marozava, S.; Probst, A.J.; Meckenstock, R.U. Groundwater Cable Bacteria Conserve Energy by Sulfur Disproportionation. ISME J. 2020, 14, 623–634. [Google Scholar] [CrossRef]
- Müller, H.; Bosch, J.; Griebler, C.; Damgaard, L.R.; Nielsen, L.P.; Lueders, T.; Meckenstock, R.U. Long-Distance Electron Transfer by Cable Bacteria in Aquifer Sediments. ISME J. 2016, 10, 2010–2019. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, L.; Risgaard-Petersen, N.; Fossing, H.; Christensen, P.; Sayama, M. Electric Currents Couple Spatially Separated Biogeochemical Processes in Marine Sediment. Nature 2010, 463, 1071–1074. [Google Scholar] [CrossRef]
- Aller, R.C.; Aller, J.J.; Zhu, Q.; Heilbrun, C.; Klingensmith, I.; Kaushik, A. Worm Tubes as Conduits for the Electrogenic Microbial Grid in Marine Sediments. Sci. Adv. 2019, 5, eaaw3651. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.; Nielsen, L.P.; Schramm, A. Cable Bacteria Associated with Long-distance Electron Transport in New England Salt Marsh Sediment. Environ. Microbiol. Rep. 2015, 7, 175–179. [Google Scholar] [CrossRef]
- Risgaard-Petersen, N.; Kristiansen, M.; Frederiksen, R.B.; Dittmer, A.; Bjerg, J.; Trojan, D.; Schreiber, L.; Damgaard, L.; Schramm, A.; Nielsen, L. Cable Bacteria in Freshwater Sediments. Appl. Environ. Microb. 2015, 81, 6003–6011. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Reimers, C.E.; Chapman, J.W. Microbiome Analyses and Presence of Cable Bacteria in the Burrow Sediment of Upogebia Pugettensis. Mar. Ecol. Prog. Ser. 2020, 648, 79–94. [Google Scholar] [CrossRef]
- Malkin, S.Y.; Meysman, F.J. Rapid Redox Signal Transmission by “Cable Bacteria” beneath a Photosynthetic Biofilm. Appl. Environ. Microb. 2015, 81, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Malkin, S.Y.; Seitaj, D.; Burdorf, L.D.W.; Nieuwhof, S.; Hidalgo-Martinez, S.; Tramper, A.; Geeraert, N.; Stigter, H.D.; Meysman, F.J.R. Electrogenic Sulfur Oxidation by Cable Bacteria in Bivalve Reef Sediments. Front. Mar. Sci. 2017, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Hermans, M.; Lenstra, W.K.; Hidalgo-Martinez, S.; van Helmond, N.A.G.M.; Witbaard, R.; Meysman, F.J.R.; Gonzalez, S.; Slomp, C.P. Abundance and Biogeochemical Impact of Cable Bacteria in Baltic Sea Sediments. Environ. Sci. Technol. 2019, 53, 7494–7503. [Google Scholar] [CrossRef]
- Seitaj, D.; Schauer, R.; Sulu-Gambari, F.; Hidalgo-Martinez, S.; Malkin, S.Y.; Burdorf, L.D.W.; Slomp, C.P.; Meysman, F.J.R. Cable Bacteria Generate a Firewall against Euxinia in Seasonally Hypoxic Basins. Proc. Natl. Acad. Sci. USA 2015, 112, 13278–13283. [Google Scholar] [CrossRef] [Green Version]
- Kessler, A.J.; Wawryk, M.; Marzocchi, U.; Roberts, K.L.; Wong, W.W.; Risgaard-Petersen, N.; Meysman, F.J.R.; Glud, R.N.; Cook, P.L.M. Cable Bacteria Promote DNRA through Iron Sulfide Dissolution. Limnol. Oceanogr. 2018, 64, 2834–2857. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.M.F.; Malkin, S.Y.; Hidalgo-Martinez, S.; Meysman, F.J.R. The Impact of Electrogenic Sulfide Oxidation on Elemental Cycling and Solute Fluxes in Coastal Sediment. Geochim. Cosmochim. Acta 2016, 172, 265–286. [Google Scholar] [CrossRef] [Green Version]
- Sulu-Gambari, F.; Seitaj, D.; Behrends, T.; Banerjee, D.; Meysman, F.J.R.; Slomp, C.P. Impact of Cable Bacteria on Sedimentary Iron and Manganese Dynamics in a Seasonally-Hypoxic Marine Basin. Geochim. Cosmochim. Acta 2016, 192, 49–69. [Google Scholar] [CrossRef]
- Sulu-Gambari, F.; Seitaj, D.; Meysman, F.J.R.; Schauer, R.; Polerecky, L.; Slomp, C.P. Cable Bacteria Control Iron–Phosphorus Dynamics in Sediments of a Coastal Hypoxic Basin. Environ. Sci. Technol. 2016, 50, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.; Risgaard-Petersen, N.; Meysman, F.J.R.; Slomp, C.P. Biogeochemical Impact of Cable Bacteria on Coastal Black Sea Sediment. Biogeosciences 2020, 17, 5919–5938. [Google Scholar] [CrossRef]
- Cornelissen, R.; Bøggild, A.; Eachambadi, R.T.; Koning, R.I.; Kremer, A.; Hidalgo-Martinez, S.; Zetsche, E.-M.; Damgaard, L.R.; Bonné, R.; Drijkoningen, J.; et al. The Cell Envelope Structure of Cable Bacteria. Front. Microbiol. 2018, 9, 3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meysman, F.J.R.; Cornelissen, R.; Trashin, S.; Bonne, R.; Martinez, S.H.; van der Veen, J.; Blom, C.J.; Karman, C.; Hou, J.-L.; Eachambadi, R.T.; et al. A Highly Conductive Fibre Network Enables Centimetre-Scale Electron Transport in Multicellular Cable Bacteria. Nat. Commun. 2019, 10, 4120. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M. Microbiology: Conductive Consortia. Nature 2015, 526, 513–514. [Google Scholar] [CrossRef]
- Schreiber, L.; Holler, T.; Knittel, K.; Meyerdierks, A.; Amann, R. Identification of the Dominant Sulfate-reducing Bacterial Partner of Anaerobic Methanotrophs of the ANME-2 Clade. Environ. Microbiol. 2010, 12, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Scheller, S.; Yu, H.; Chadwick, G.; McGlynn, S.; Orphan, V. Artificial Electron Acceptors Decouple Archaeal Methane Oxidation from Sulfate Reduction. Science 2016, 351, 703–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Reimers, C.E.; Alleau, Y. Inducing the Attachment of Cable Bacteria on Oxidizing Electrodes. Biogeosciences 2020, 17, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Reimers, C.E.; Li, C.; Graw, M.F.; Schrader, P.S.; Wolf, M. The Identification of Cable Bacteria Attached to the Anode of a Benthic Microbial Fuel Cell: Evidence of Long Distance Extracellular Electron Transport to Electrodes. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Reimers, C.E.; Alleau, Y.; Bauer, J.E.; Delaney, J.; Girguis, P.R.; Schrader, P.S.; Stecher, H.A. Redox Effects on the Microbial Degradation of Refractory Organic Matter in Marine Sediments. Geochim. Cosmochim. Acta 2013, 121, 582–598. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Oliveros, J.C.V. An Interactive Tool for Comparing Lists with Venn’s Diagrams; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lücker, S.; Steger, D.; Kjeldsen, K.U.; MacGregor, B.J.; Wagner, M.; Loy, A. Improved 16S RRNA-Targeted Probe Set for Analysis of Sulfate-Reducing Bacteria by Fluorescence in Situ Hybridization. J. Microbiol. Meth. 2007, 69, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, N.M.J.; Zetsche, E.-M.; Hidalgo-Martinez, S.; Middelburg, J.J.; Meysman, F.J.R. Mineral Formation Induced by Cable Bacteria Performing Long-Distance Electron Transport in Marine Sediments. Biogeosciences 2019, 16, 811–829. [Google Scholar] [CrossRef] [Green Version]
- Geelhoed, J.S.; van de Velde, S.J.; Meysman, F.J.R. Quantification of Cable Bacteria in Marine Sediments via QPCR. Front. Microbiol. 2020, 11, 1506. [Google Scholar] [CrossRef]
- Bjerg, J.T.; Damgaard, L.R.; Holm, S.A.; Schramm, A.; Nielsen, L.P. Motility of Electric Cable Bacteria. Appl. Environ. Microb. 2016, 82, 3816–3821. [Google Scholar] [CrossRef] [Green Version]
- Ryckelynck, N.; Stecher, H.A.; Reimers, C.E. Understanding the Anodic Mechanism of a Seafloor Fuel Cell: Interactions between Geochemistry and Microbial Activity. Biogeochemistry 2005, 76, 113–139. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic Substances as Electron Acceptors for Microbial Respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Organic Matter Mineralization with Reduction of Ferric Iron in Anaerobic Sediments. Appl. Environ. Microb. 1986, 51, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, T.M.; O’Loughlin, E.J.; Mishra, B.; DiChristina, T.J.; Kemner, K.M. Sulfur-Mediated Electron Shuttling during Bacterial Iron Reduction. Science 2014, 344, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Dam, A.; Marshall, I.P.G.; Risgaard-Petersen, N.; Burdorf, L.D.W.; Marzocchi, U. Effect of Salinity On Cable Bacteria Species Composition And Diversity. Environiron. Microbiol. 2021, 23, 2605–2616. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Nelson, W.G.; Boese, B.L.; DeWitt, T.H.; Eldridge, P.M.; Kaldy, J.E.; Il, H.L.; Power, J.H.; Young, D.R. An Approach to Developing Nutrient Criteria for Pacific Northwest. Estuaries—A Case Study of Yaquina Estuary, Oregon; US EPA Office of Research and Development: Washington, DC, USA, 2007. Available online: https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=471482&Lab=NHEERL (accessed on 7 October 2021).
- Kjeldsen, K.U.; Schreiber, L.; Thorup, C.A.; Boesen, T.; Bjerg, J.T.; Yang, T.; Dueholm, M.S.; Larsen, S.; Risgaard-Petersen, N.; Nierychlo, M.; et al. On the Evolution and Physiology of Cable Bacteria. Proc. Natl. Acad. Sci. USA 2019, 116, 19116–19125. [Google Scholar] [CrossRef] [Green Version]
- Marzocchi, U.; Trojan, D.; Larsen, S.; Meyer, R.L.; Revsbech, N.P.; Schramm, A.; Nielsen, L.P.; Risgaard-Petersen, N. Electric Coupling between Distant Nitrate Reduction and Sulfide Oxidation in Marine Sediment. ISME J. 2014, 8, 1682–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, M.O.; Deutzmann, J.; Spormann, A.; Rotaru, A.-E. Cultivating Electroactive Microbesfrom Field to Bench. Nanotechnology 2020, 31, 174003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Menor, E.; Gimeno-Valero, H.; Pascual, J.; Peretó, J.; Porcar, M. High Culturable Bacterial Diversity from a European Desert: The Tabernas Desert. Front. Microbiol. 2021, 11, 583120. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Reimers, C.E.; Alleau, Y. Using Oxidative Electrodes to Enrich Novel Members in the Desulfobulbaceae Family from Intertidal Sediments. Microorganisms 2021, 9, 2329. https://doi.org/10.3390/microorganisms9112329
Li C, Reimers CE, Alleau Y. Using Oxidative Electrodes to Enrich Novel Members in the Desulfobulbaceae Family from Intertidal Sediments. Microorganisms. 2021; 9(11):2329. https://doi.org/10.3390/microorganisms9112329
Chicago/Turabian StyleLi, Cheng, Clare E. Reimers, and Yvan Alleau. 2021. "Using Oxidative Electrodes to Enrich Novel Members in the Desulfobulbaceae Family from Intertidal Sediments" Microorganisms 9, no. 11: 2329. https://doi.org/10.3390/microorganisms9112329
APA StyleLi, C., Reimers, C. E., & Alleau, Y. (2021). Using Oxidative Electrodes to Enrich Novel Members in the Desulfobulbaceae Family from Intertidal Sediments. Microorganisms, 9(11), 2329. https://doi.org/10.3390/microorganisms9112329