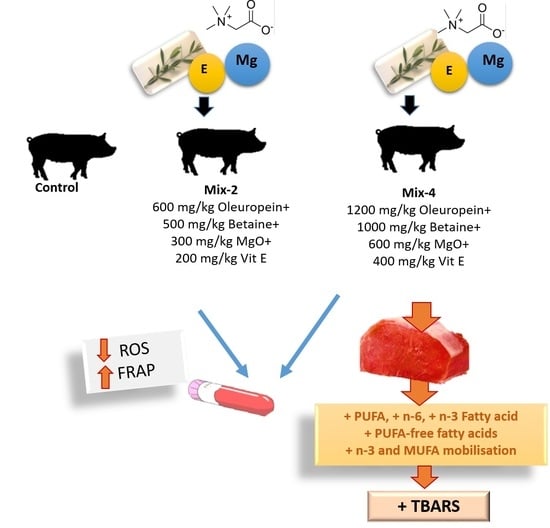
Supplementation Effect of Oleuropein Extract Combined with Betaine, Magnesium, and Vitamin E on Pigs’ Performance and Meat Quality Characteristics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Diets and Sample Collection
2.2. Laboratory Analysis
2.2.1. Biochemical Parameters and Antioxidant Capacity in Serum Samples
2.2.2. Tocopherol Content of Serum and Muscle Samples
2.2.3. Drip Loss in Muscle Samples
2.2.4. TBARS of Serum and Muscle Samples
2.2.5. Texture Parameters Measurement
2.2.6. Lipid Fractions and Fatty Acid Profile of Intramuscular Fat
2.2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ortuño, J.; Serrano, R.; Jordán, M.J.; Bañón, S. Relationship between antioxidant status and oxidative stability in lamb meat reinforced with dietary rosemary diterpenes. Food Chem. 2016, 190, 1056–1063. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Hopkins, D.L.; Giri, K.; Jacobs, J.L.; Plozza, A.; Lewendoski, P.; Bekhit, A. The use of oxidative stress biomarkers in live animals (In vivo) to predict meat quality deterioration postmortem (in vitro) caused by changes in muscle biochemical components. J. Anim. Sci. 2017, 95, 3012–3024. [Google Scholar] [CrossRef]
- Dugan, M.E.R.; Aalhus, J.L.; Uttaro, B. Nutritional Manipulation of pork quality. Current Opportunities. Adv. Pork Product. 2004, 15, 237–243. [Google Scholar]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Rey, A.I.; de-Cara, A.; Calvo, L.; Puig, P.; Hechavarría, T. Changes in Plasma Fatty Acids, Free Amino Acids, Antioxidant Defense, and Physiological Stress by Oleuropein Supplementation in Pigs Prior to Slaughter. Antioxidants 2020, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Rey, A.I.; de-Cara, A.; Segura, J.F.; Martí, P.; Hechavarría, T.; Calvo, L. Dietary oleuropein extract supplementation and their combination with α-tocopheryl acetate and selenium modifies free fatty acid profile and improves stability of pork. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Nieto, G.; Pateiro, M.; Lorenzo, J.M. Phenolic Compounds Obtained from Olea europaea By-Products and their Use to Improve the Quality and Shelf Life of Meat and Meat Products—A Review. Antioxidants 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; López-Bote, C.J.; Litta, G. Effects of dietary vitamin E (DL-α-tocopheryl acetate) and vitamin C combination on piglets oxidative status and immune response at weaning. J. Anim. Feed Sci. 2017, 2, 226–235. [Google Scholar] [CrossRef]
- Rey, A.I.; López-Bote, C.J.; Kerry, J.P.; Lynch, P.B.; Buckley, D.J.; Morrissey, P.A. Modification of lipid composition and oxidation in porcine muscle and muscle microsomes as affectd by dietary supplementation of n-3 with either n-9 or n-6 fatty acids and α-tocopheryl acetate. Anim. Feed Sci. Technol. 2004, 113, 223–238. [Google Scholar] [CrossRef]
- Calvo, L.; Toldrá, F.; Aristoy, M.C.; Lopez-Bote, C.J.; Rey, A.I. Effect of dietary organic selenium on muscle proteolytic activity and water-holding capacity in pork. Meat Sci. 2016, 121, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Olivares, A.; Rey, A.I.; Daza, A.; López-Bote, C.J. High dietary vitamin A interferes with tissue -tocopherol concentrations in fattening pigs: A study that examines administration and withdrawal times. Animal 2009, 3, 1264–1270. [Google Scholar] [CrossRef]
- Ayuso, M.; Ovilo, C.; Fernández, A.; Nuñez, Y.; Isabel, B.; Daza, A.; López-Bote, C.J.; Rey, A.I. Effects of dietary vitamin A supplementation or restriction and its timing on retinol and α-tocopherol accumulation and gene expression in heavy pigs. Anim. Feed Sci. Technol. 2015, 202, 62–74. [Google Scholar] [CrossRef]
- Lipinski, K.; Szramko, E.; Jeroch, H.; Matusevicious, P. Effects of betaine on Energy utilization in growing pigs—A review. Ann. Anim. Sci. 2012, 12, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.; Babazadeh, D.; Naveed, M.; Arain, M.A.; Hassan, F.U.I.; Chao, S. Reconsidering betaine as a natural anti-heat stress agent in poultry industry: A review. Trop. Anim. Health Prod. 2017, 49, 1329–1338. [Google Scholar] [CrossRef]
- Eklund, M.; Bauer, E.; Wamatu, J.; Mosenthin, R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005, 18, 31–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.-H.; Hur, S.J.; Park, G.B.; Joo, S.T. Effects of dietary glycine betaine on blood characteristics and pork quality. J. Muscle Foods 2010, 21, 87–101. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Wang, X.; Wang, Y.; Feng, J. Betaine affects muscle lipid metabolism via regulating the fatty acid uptake and oxidation in finishing pig. J. Anim. Sci. Biotechnol. 2017, 8, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. Growth performance and characterization of meat quality of broiler chickens supplemented with betaine and antioxidants under cyclic heat stress. Antioxidants 2019, 8, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, K.; Stasiewicz, M.; Purwin, C.; Zuk-Golaszewska-Zuk, K. Effects of magnesium on pork quality. J. Elem. 2011, 2, 325–337. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine; The National Academic Press: Washington, DC, USA, 2012.
- Nogueira, T.M.; Mendes, E.; Da Matta, I.; Borges, P.C.; Bolleta, A.G.; Marcal, J.O.; Carvalho, F.P.; Faria, P.B. Lipid profile and quality of meat from finishing pig supplemented with minerals. Food Sci. Technol. 2019, 39, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Verma, H.; Garg, R. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633. [Google Scholar] [CrossRef] [PubMed]
- BOE. RD 53/2013, de 21 de octubre por la que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia, Spain. Boletín Oficial del Estado 2013, 252, 34367–34391. [Google Scholar]
- EC. Council Regulation (EC) No 2009/1099/CE of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Union 2009, 303, 1–27. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Method Enzymol. 1999, 299, 15–27. [Google Scholar]
- Rey, A.I.; Daza, A.; López-Carrasco, C.; López-Bote, C.J. Quantitative study of the alpha- and gamma-tocopherols accumulation in muscle and backfat from Iberian pigs kept free-range as affected by time of free-range feeding or weight gain. Anim. Sci. 2006, 82, 901–908. [Google Scholar] [CrossRef]
- Amazan, D.; Cordero, G.; López-Bote, C.J.; Lauridsen, C.; Rey, A.I. Effects of oral micellized natural vitamin E (D-α-tocopherol) v. synthetic vitamin E (DL-α-tocopherol) in feed on α-tocopherol levels, stereoisomer distribution, oxidative stress and the immune response in piglets. Animal 2014, 8, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Segura, J.; López-Bote, C.J. A laboratory efficient method for intramuscular fat analysis. Food Chem. 2014, 145, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Ruíz, J.; Antequera, T.; Andrés, A.I.; Petrón, M.J.; Muriel, E. Improvement of a solid phase extraction method for analysis of lipid fractions in muscle foods. Anal. Chim. Acta 2004, 520, 201–205. [Google Scholar] [CrossRef]
- Sarika, S.; Toptas, S. Effects of dietary oleuropein supplementation on growth performance, serum lipid concentrations and lipid oxidation of Japonese quails. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1176–1186. [Google Scholar] [CrossRef]
- Tarsitano, M.A.; Bridi, A.M.; da Silva, C.A.; Constantino, C.; Andreo, N.; Dalto, D.B. Magnesium supplementation in swine finishing stage: Performance, carcass characteristics and meat quality. Semina Ciencias Agrarias 2013, 34, 3105–3118. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Fígares, I.; Wray-Cahen, D.; Steele, N.C.; Campbell, R.G.; Hall, D.D.; Virtanen, E.; Caperna, T.J. Effect of dietary betaine on nutrient utilization and partitioning in the young growing feed-restricted pig. J. Anim. Sci. 2002, 80, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Siljander-Rasi, H.; Peuranen, S.; Tiihonen, K.; Virtanen, E.; Kettunen, H.; Alaviuhkola, T.; Simmins, P.H. Effect of equi-molar dietary betaine and choline addition on performance, carcass quality and physiological parameters of pigs. Anim. Sci. 2003, 76, 55–62. [Google Scholar] [CrossRef]
- Sales, J. A meta-analysis of the effects of dietary betaine supplementation on finishing performance and carcass characteristics of pigs. Anim. Feed Sci. Technol. 2011, 165, 68–78. [Google Scholar] [CrossRef]
- Hadrich, F.; Garcia, M.; Maalej, A.; Modes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Moazzami, A.A.; Andersson, R.; Kamal-Eldin, A. Changes in the metabolic profile of rat liver after α-tocopherol deficiency as revealed by metabolomics analysis. NMR Biomed. 2011, 24, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, W.; Yao, H.; Sun, W.; Zhou, Q.; Cail, L. Association of serum and urinary magnesium with the pre-diabetes, diabetes and diabetic complications in the Chineses Northeast population. PLoS ONE 2013, 8, e56750. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.K.; Melo, A.E.; Domingueti, C.P. Association between reduced serum levels of magnesium and the presence of poor glycemic control and complications in type 1 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 127–134. [Google Scholar] [CrossRef]
- Shah, A.M.; Ma, J.; Wang, Z.; Zou, H.; Hu, R.; Peng, Q. Betaine Supplementation Improves the Production Performance, Rumen Fermentation, and Antioxidant Profile of Dairy Cows in Heat Stress. Animals 2020, 10, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahi, P.; Alirezaei, M.; Kheradmand, A.; Rocky, A. Betaine: A Promising Micronutrient in Diet Intervention for Ameliorating Maternal Blood Biochemical Alterations in Gestational Diabetes Mellitus. Int. J. Pept. Res. Ther. 2020, 26, 1177–1184. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; de Oliveira, A.R.S.; Cruz, K.J.L.; Dias, T.M.D.; De Assis, R.C.; Colli, C.; Marreiro, D.D. Magnesium Status and Its Association with Oxidative Stress in Obese Women. Biol. Trace Elem. Res. 2017, 175, 306–311. [Google Scholar] [CrossRef]
- Monteiro, C.P.; Matias, C.N.; Bicho, M.; Santa-Clara, H.; Laires, M.J. Coordination between antioxidant defences might be partially modulated by magnesim status. Magnes. Res. 2016, 29, 161–167. [Google Scholar]
- Giris, M.; Dogru-Abbasoglu, S.; Soluk-Tekkesin, M.; Olgac, V.; Uysal, M. Effect of betaine treatment on the regression of existing hepatic triglyceride accumulation and oxidative stress in rats fed on high fructose diet. Gen. Physiol. Biophys. 2018, 37, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Alirezaei, M.; Jelodar, G.; Ghayemi, Z. Antioxidant Defense of Betaine against Oxidative Stress Induced by Ethanol in the Rat Testes. Int. J. Pept. Res. Ther. 2012, 18, 239–247. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Barbosa, S.; Pinheiro, V.; Mourão, J.L.; Outor-Monteiro, D. The effect of olive leaves supplementation on the feed digestibility, growth performance of pigs and quality of pork meat. Meat Sci. 2009, 82, 438–443. [Google Scholar] [CrossRef]
- Alonso, V.; Provincial, L.; Gil, M.; Guillén, E.; Roncalés, P.; Beltrán, J.A. The impact of short-term feeding of magnesium supplements on the quality of pork packaged in modified atmosphere. Meat Sci. 2012, 90, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. Dietary Betaine Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status and Meat Quality in Broiler Chickens. Agriculture 2020, 10, 176. [Google Scholar] [CrossRef]
- Espín, J.C.; Gonzalez-Barrio, R.; Cerdá, B.; López-Bote, C.; Rey, A.I.; Tomas-Barberán, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef]
- Muñoz-Gonzalez, I.; Chamorro, S.; Perez-Jiménez, J.; Lopez-Andrés, P.; Álvarez-Acero, I.; Herrero, A.M.; Nardoia, M.; Brenes, A.; Viveros, A.; Arija, I.; et al. Phenolic Metabolites in Plasma and Thigh Meat of Chickens Supplemented with Grape Byproducts. J. Agric. Food Chem. 2019, 67, 4463–4471. [Google Scholar] [CrossRef] [PubMed]
- Lahucky, R.; Küchenmeister, U.; Bahelka, I.; Vasicek, D.; Liptaj, T.; Ender, K. The effect of dietary magnesium oxide supplementation on postmortem 31P NMR spectroscopy parameters, rate of Ca2+ uptake and ATPase activity of M. longissimus dorsi and meat quality of heterozygous and normal on malignant hyperthermia pigs. Meat Sci. 2004, 67, 365–370. [Google Scholar] [CrossRef]
- Mattews, J.O.; Southerm, L.L.; Bidner, T.D.; Persica, M.A. Effects of betaine, pen space, and slaughter handling method on growth performance, carcass traits, and pork quality of finishing barrows. J. Anim. Sci. 2001, 79, 967–974. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, D.; Wang, Y. The effects of betaine on weanling piglets and its mechanism. J. Zhejiang Univ. Sci. 1999, 25, 543–546. [Google Scholar]
- Yang, H.S.; Lee, J.I.; Joo, S.T.; Park, G.B. Effects of dietary glycine betaine on growth and pork quality of finishing pigs. Asian-Australas. J. Anim. Sci. 2009, 22, 706–711. [Google Scholar] [CrossRef]
- Zabaras-Krick, B. Betaine improves energy utilisation. Int. Pig Topics 1997, 12, 12–14. [Google Scholar]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Raclot, T.; Groscolas, R. Differential mobilization of white adipose tissue fatty acids according to chain length, unsaturation, and positional isomerism. J. Lipid Res. 1993, 34, 1515–1526. [Google Scholar] [CrossRef]
- Rey, A.I.; Amazan, D.; Cordero, G.; Olivares, A.; López-Bote, C.J. Lower oral doses of micellized α-tocopherol compared to α-tocophery acetate in feed modify fatty acid profiles and improve oxidative status in pigs. Int. J. Vit. Nutr. Res. 2014, 84, 229–243. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Bara, F.F.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Rey, A.I.; Segura, J.; Castejón, D.; Fernández-Valle, M.E.; Cambero, I.; Calvo, L. Vitamin D3 supplementation in drinking water prior to slaughter improves oxidative status, physiological stress and quality of pork. Antioxidants 2020, 9, 559. [Google Scholar] [CrossRef]

| Nutrients | % |
|---|---|
| Protein | 15.08 |
| Crude fat | 6.69 |
| Crude fiber | 5.18 |
| Neutral detergent fiber (NDF) | 14.87 |
| Starch | 41.86 |
| Sugar | 3.12 |
| Ash | 4.91 |
| Calcium | 0.60 |
| Total phosphorous | 0.55 |
| Net energy (Mcal/kg) | 2.44 |
| Pigs’ Performances | Treatments 1 | ||||
|---|---|---|---|---|---|
| Control | MIX-2 | MIX-4 | SEM 2 | p Value | |
| Initial body weight, kg | 86.44 | 87.08 | 87.58 | 2.671 | 0.9567 |
| Body weight at day 34, kg | 119.52 | 120.76 | 119.87 | 2.075 | 0.9128 |
| ADWG total, g/d | 0.99 | 0.99 | 0.96 | 0.032 | 0.8054 |
| ADFI total, g/d | 3.05 | 3.02 | 3.01 | 0.098 | 0.9388 |
| FCR total, g/g | 3.09 | 3.05 | 3.13 | 0.158 | 0.9312 |
| Carcass Yield | |||||
| Lean, % | 51.79 | 52.26 | 53.27 | 1.059 | 0.6027 |
| Fat thickness at 3–4 rib, mm | 25.48 | 25.00 | 24.02 | 1.127 | 0.6509 |
| Lean thickness at 3–4 rib, mm | 55.99 | 55.65 | 56.60 | 0.934 | 0.7659 |
| Fat thickness of ham, mm | 33.61 | 33.58 | 32.39 | 1.346 | 0.7639 |
| Lean percentage of ham | 65.64 | 66.14 | 66.48 | 0.906 | 0.8038 |
| Lean percentage of loin | 57.70 | 57.96 | 58.39 | 0.694 | 0.7732 |
| Ham Weight, kg | 11.72 | 11.67 | 11.69 | 0.100 | 0.9406 |
| Belly Weight, kg | 4.44 | 4.46 | 4.45 | 0.056 | 0.9452 |
| Shoulder Weight, kg | 6.60 | 6.64 | 6.65 | 0.061 | 0.8168 |
| Loin Weight, kg | 8.19 | 8.14 | 8.14 | 0.086 | 0.8893 |
| Ham Lean, % | 68.09 | 68.22 | 68.52 | 0.474 | 0.8100 |
| Belly Lean, % | 54.26 | 54.55 | 54.95 | 0.680 | 0.7705 |
| Shoulder Lean, % | 65.28 | 65.39 | 65.64 | 0.420 | 0.8233 |
| Loin Lean, % | 57.70 | 57.96 | 58.39 | 0.694 | 0.7732 |
| Treatments 1 | SEM 2 | p Value | |||
|---|---|---|---|---|---|
| Control | MIX-2 | MIX-4 | |||
| Serum parameters | |||||
| Glucose, mg/100 mL | 90.40 a | 92.57 a | 78.56 b | 3.692 | 0.0308 |
| α-tocopherol, µg/mL | 4.95 b | 7.87 a | 9.04 a | 0.497 | 0.0001 |
| TBARS, mmoles/L | 0.0043 a | 0.0041 ab | 0.0035 b | 0.000 | 0.0704 |
| FRAP, µM | 94.70 b | 135.38 a | 139.98 a | 9.263 | 0.0026 |
| Muscle composition | |||||
| Drip loss, % | 9.673 | 7.754 | 8.374 | 0.523 | 0.1479 |
| Moisture, % | 73.713 | 74.040 | 74.306 | 0.226 | 0.1966 |
| Intramuscular fat, % | 3.231 | 3.004 | 2.724 | 0.274 | 0.4353 |
| α-tocopherol, ug/g | 2.333 b | 3.004 ab | 3.373 a | 0.177 | 0.0011 |
| Meat quality traits | |||||
| Drip loss, % | 9.673 | 7.754 | 8.374 | 0.523 | 0.1479 |
| TBARS (mg MDA/kg) | |||||
| Day 0 | 0.116 | 0.126 | 0.126 | 0.010 | 0.6070 |
| Day 4 | 0.269 | 0.289 | 0.307 | 0.031 | 0.5188 |
| Day 7 | 0.228 b | 0.307 ab | 0.343 a | 0.039 | 0.0330 |
| Texture parameters | |||||
| Hardness, N | 50.828 | 42.258 | 44.198 | 4.005 | 0.3000 |
| Adhesiveness, Nxs | −0.452 | −0.468 | −0.480 | 0.031 | 0.8166 |
| Springiness, m | 0.001 | 0.001 | 0.001 | 0.000 | 0.4945 |
| Cohesiveness | 0.421 | 0.413 | 0.417 | 0.013 | 0.9142 |
| Gumminess, N | 21.340 | 17.287 | 18.297 | 1.650 | 0.2135 |
| Chewiness, J | 0.023 | 0.027 | 0.018 | 0.006 | 0.5423 |
| Treatments 1 | SEM 2 | p Value | |||
|---|---|---|---|---|---|
| Control | MIX-2 | MIX-4 | |||
| C14:0 | 1.46 | 1.40 | 1.44 | 0.051 | 0.7313 |
| C16:0 | 24.73 | 24.79 | 24.58 | 0.359 | 0.9330 |
| C16:1n9 | 0.18 | 0.19 | 0.19 | 0.010 | 0.5158 |
| C16:1n7 | 3.86 | 3.68 | 3.69 | 0.193 | 0.7656 |
| C17:0 | 0.19 | 0.20 | 0.17 | 0.008 | 0.1494 |
| C17:1 | 0.19 | 0.20 | 0.19 | 0.010 | 0.6031 |
| C18:0 | 12.81 | 13.11 | 12.96 | 0.359 | 0.4834 |
| C18:1n9 | 46.30 a | 46.52 a | 44.65 b | 0.430 | 0.0092 |
| C18:1n7 | 3.58 | 3.28 | 3.35 | 0.143 | 0.2576 |
| C18:2n6 | 5.06 b | 5.01 b | 6.52 a | 0.207 | 0.0001 |
| C18:3n3 | 0.27 | 0.26 | 0.24 | 0.012 | 0.2889 |
| C20:0 | 0.24 a | 0.22 ab | 0.20 b | 0.009 | 0.0185 |
| C20:1n9 | 0.82 | 0.81 | 0.77 | 0.036 | 0.7227 |
| C20:3n6 | 0.06 b | 0.08 b | 0.11 a | 0.009 | 0.0006 |
| C20:4n6 | 0.19 b | 0.21 b | 0.69 a | 0.015 | 0.0001 |
| C20:5n3 | 0.02 a | 0.02 b | 0.01 b | 0.002 | 0.0001 |
| C22:4 | 0.02 b | 0.01 b | 0.15 a | 0.005 | 0.0001 |
| C22:5n3 | 0.02 a | 0.01 c | 0.01 b | 0.001 | 0.0001 |
| C22:6n3 | 0.02 b | 0.01 b | 0.09 a | 0.004 | 0.0001 |
| ∑SAT 3 | 39.73 | 39.37 | 39.66 | 0.548 | 0.5999 |
| ∑MUFA 4 | 54.73 a | 54.88 a | 52.74 b | 0.477 | 0.0064 |
| ∑PUFA 5 | 5.54 b | 5.75 b | 7.60 a | 0.231 | 0.0001 |
| ∑n-6 | 5.22 b | 5.44 b | 7.26 a | 0.222 | 0.0001 |
| ∑n-3 | 0.32 ab | 0.32 b | 0.34 a | 0.014 | 0.0095 |
| ∑n-6/∑n-3 | 16.17 c | 17.37 b | 21.51 a | 0.576 | 0.0001 |
| Treatments 1 | SEM 2 | p Value | |||
|---|---|---|---|---|---|
| Control | MIX-2 | MIX-4 | |||
| C14:0 | 1.46 | 1.42 | 1.39 | 0.043 | 0.4104 |
| C16:0 | 25.91 a | 25.23 ab | 23.88 b | 0.412 | 0.0034 |
| C16:1n9 | 0.37 ab | 0.46 a | 0.35 b | 0.027 | 0.0159 |
| C16:1n7 | 2.85 | 2.88 | 3.18 | 0.151 | 0.1896 |
| C17:0 | 0.41 | 0.34 | 0.39 | 0.023 | 0.0912 |
| C17:1 | 0.19 ab | 0.16 b | 0.21 a | 0.015 | 0.0095 |
| C18:0 | 17.66 | 17.08 | 17.19 | 0.634 | 0.8020 |
| C18:1n9 | 35.47 | 36.29 | 36.24 | 0.726 | 0.2736 |
| C18:1n7 | 6.00 | 6.14 | 6.37 | 0.208 | 0.4128 |
| C18:2n6 | 5.84 | 6.51 | 6.32 | 0.280 | 0.6219 |
| C18:3n3 | 0.67 ab | 0.55 b | 0.71 a | 0.037 | 0.0127 |
| C20:0 | 0.28 b | 0.24 b | 0.33 a | 0.011 | 0.0001 |
| C20:1n9 | 0.75 ab | 0.70 b | 0.76 a | 0.035 | 0.0116 |
| C20:3n6 | 0.14 b | 0.14 b | 0.23 a | 0.013 | 0.0001 |
| C20:4n6 | 1.44 | 1.33 | 1.72 | 0.133 | 0.1629 |
| C20:5n3 | 0.16 | 0.15 | 0.17 | 0.037 | 0.9809 |
| C22:4n6 | 0.11 b | 0.13 b | 0.16 a | 0.013 | 0.0034 |
| C22:5n3 | 0.08 b | 0.05 b | 0.11 a | 0.010 | 0.0003 |
| C22:6n3 | 0.20 b | 0.19 b | 0.30 a | 0.021 | 0.0003 |
| ∑SAT 3 | 45.72 | 44.31 | 43.18 | 0.765 | 0.1166 |
| ∑MUFA 4 | 45.63 | 46.63 | 47.11 | 0.839 | 0.3019 |
| ∑PUFA 5 | 8.65 b | 9.06 ab | 9.72 a | 0.367 | 0.0654 |
| ∑n-6 | 1.69 b | 1.60 b | 2.11 a | 0.141 | 0.0339 |
| ∑n-3 | 0.58 b | 0.53 b | 0.81 a | 0.052 | 0.0009 |
| Treatments 1 | SEM 2 | p Value | |||
|---|---|---|---|---|---|
| Control | MIX-2 | MIX-4 | |||
| C14:0 | 3.12 | 3.31 | 3.26 | 0.152 | 0.9216 |
| C16:0 | 23.36 ab | 24.25 a | 23.07 b | 0.351 | 0.0245 |
| C16:1n9 | 1.53 a | 1.62 a | 1.32 b | 0.051 | 0.0001 |
| C16:1n7 | 0.73 b | 0.80 b | 1.07 a | 0.069 | 0.0002 |
| C17:0 | 0.45 b | 0.45 b | 1.57 a | 0.114 | 0.0001 |
| C17:1 | 0.28 b | 0.28 b | 1.22 a | 0.106 | 0.0001 |
| C18:0 | 6.89 | 7.13 | 6.88 | 0.209 | 0.2522 |
| C18:1n9 | 11.78 | 12.22 | 11.42 | 0.425 | 0.5524 |
| C18:1n7 | 1.82 ab | 1.73 a | 1.83 a | 0.053 | 0.0549 |
| C18:2n6 | 35.62 a | 32.66 b | 33.41 b | 0.503 | 0.0021 |
| C18:3n3 | 0.38 a | 0.35 ab | 0.33 b | 0.010 | 0.0438 |
| C20:0 | 0.07 | 0.06 | 0.07 | 0.005 | 0.4129 |
| C20:1n9 | 0.17 | 0.17 | 0.16 | 0.008 | 0.1199 |
| C20:3n6 | 1.06 ab | 1.10 a | 1.04 b | 0.028 | 0.0583 |
| C20:4n6 | 9.44 | 10.30 | 9.99 | 0.272 | 0.5264 |
| C20:5n3 | 0.25 | 0.26 | 0.25 | 0.009 | 0.9186 |
| C22:4n6 | 1.72 | 1.84 | 1.62 | 0.064 | 0.3686 |
| C22:5n3 | 1.04 | 1.13 | 1.11 | 0.033 | 0.6494 |
| C22:6n3 | 0.29 | 0.34 | 0.39 | 0.030 | 0.691 |
| ∑SAT 3 | 33.90 b | 35.20 a | 34.84 ab | 0.335 | 0.0517 |
| ∑MUFA 4 | 16.31 b | 16.82 ab | 17.01 a | 0.463 | 0.0563 |
| ∑PUFA 5 | 49.50 a | 47.64 b | 47.76 b | 0.515 | 0.0074 |
| ∑n-6 | 12.50 | 13.58 | 13.04 | 0.297 | 0.3222 |
| ∑n-3 | 1.58 | 1.73 | 1.74 | 0.057 | 0.6231 |
| ∑n-6/∑n-3 | 7.96 | 7.89 | 7.56 | 0.187 | 0.6089 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, A.I.; Puig, P.; Cardozo, P.W.; Hechavarría, T. Supplementation Effect of Oleuropein Extract Combined with Betaine, Magnesium, and Vitamin E on Pigs’ Performance and Meat Quality Characteristics. Animals 2021, 11, 443. https://doi.org/10.3390/ani11020443
Rey AI, Puig P, Cardozo PW, Hechavarría T. Supplementation Effect of Oleuropein Extract Combined with Betaine, Magnesium, and Vitamin E on Pigs’ Performance and Meat Quality Characteristics. Animals. 2021; 11(2):443. https://doi.org/10.3390/ani11020443
Chicago/Turabian StyleRey, Ana I., Patricia Puig, Paul William Cardozo, and Teresa Hechavarría. 2021. "Supplementation Effect of Oleuropein Extract Combined with Betaine, Magnesium, and Vitamin E on Pigs’ Performance and Meat Quality Characteristics" Animals 11, no. 2: 443. https://doi.org/10.3390/ani11020443
APA StyleRey, A. I., Puig, P., Cardozo, P. W., & Hechavarría, T. (2021). Supplementation Effect of Oleuropein Extract Combined with Betaine, Magnesium, and Vitamin E on Pigs’ Performance and Meat Quality Characteristics. Animals, 11(2), 443. https://doi.org/10.3390/ani11020443