Influence of Sunlight on Vitamin D and Health Status in Green (Chelonia mydas) Sea Turtles with Fibropapillomatosis
Abstract
:Simple Summary
Abstract
1. Introduction
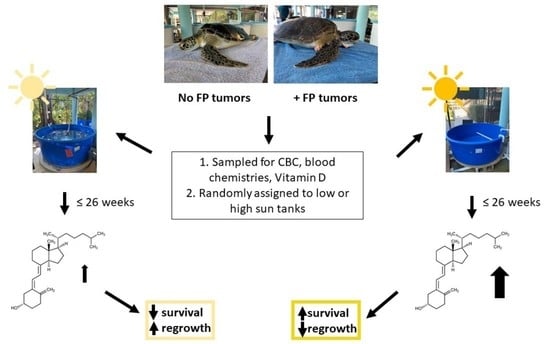
2. Materials and Methods
2.1. GLNC Candidate Selection and Care
2.2. IRG Candidate Selection and Sample Collection
2.3. UV Light Treatment
2.4. Blood Sample Collection
2.5. Post-Surgical Monitoring for Regrowth
2.6. Body Condition Index Calculations
2.7. Statistical Analysis
3. Results
3.1. Hematology and Blood Chemistry Parameters
3.2. UV Light Exposure Conditions
3.3. Vitamin D, Parathyroid Hormone, and Ionized Calcium
3.4. Observations on Regrowth and Survival
4. Discussion
4.1. Hematology and Blood Chemistry Parameters
4.2. Baseline Vitamin D Levels
4.3. Regrowth, Viral Load, and Survival
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, G.M.; Coates, C.W. Fibro-epithelial growths of the skin in large marine turtles, Chelonia mydas (Linnaeus). Zoologica 1938, 23, 93–98. [Google Scholar] [CrossRef]
- Harshbarger, J.C. Sea turtle fibropapilloma cases in the registry of tumors in lower animals. In Research Plan for Marine Turtle Fibropapilloma: Results of a December 1990 Workshop; Balazs, G.H., Pooley, S.G., Eds.; NOAA Technical Memorandum: Washington, DC, USA, 1991; NTIS No. PB-91-170258. [Google Scholar]
- Barragan, A.R.; Sarti, M.L. A possible case of fibropapilloma in Kemp’s Ridley turtle (Lepidochelys kempii). Mar. Turt. Newsl. 1994, 67, 27. [Google Scholar]
- D’Amato, A.F.; Moraes-Neto, M. First documentation of fibropapillomas verified by histopathology in Eretmochelys imbricata. Mar. Turt. Newsl. 2000, 89, 12–13. [Google Scholar]
- Aguirre, A.A.; Spraker, T.R.; Chaves, A.; Toit, L.; Eure, W.; Balazs, G.H. Pathology of fibropapillomatosis in Olive Ridley turtles Lepidochelys olivacea nesting in Costa Rica. J. Aquat. Anim. Health 1999, 11, 283–289. [Google Scholar] [CrossRef]
- Limpus, C.J.; Couper, P.J.; Couper, K.L.D. Crab Island revisited: Reassessment of the world’s largest Flatback turtle rookery after twelve years. Mem. Queensl. Mus. 1993, 33, 277–289. [Google Scholar]
- Jones, K.; Ariel, E.; Burgess, G.; Read, M. A review of fibropapillomatosis in Green turtles (Chelonia mydas). Vet. J. 2016, 212, 48–57. [Google Scholar] [CrossRef]
- Jones, A.G. Sea Turtles: Old Viruses and New Tricks. Curr. Biol. 2004, 14, R842–R843. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.J.; Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Arch. Virol. 2012, 157, 1411–1422. [Google Scholar] [CrossRef] [Green Version]
- Page-Karjian, A.; Norton, T.M.; Ritchie, B.; Brown, C.; Mancia, C.; Jackwood, M.; Gottdenker, N.L. Quantifying chelonid herpesvirus 5 in symptomatic and asymptomatic rehabilitating green sea turtles. Endang. Species Res. 2015, 28, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Milton, S.L.; Lutz, P.L. Physiological and genetic responses to environmental stress. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Wyneken, J., Eds.; CRC Press: Boca Raton, FL, USA, 2003; Volume 2, pp. 163–197. [Google Scholar]
- Aguirre, A.A.; Lutz, P.L. Marine turtles as sentinels of ecosystem health: Is fibropapillomatosis an indicator? EcoHealth 2004, 1, 275–283. [Google Scholar] [CrossRef]
- Foley, A.M.; Schroeder, B.A.; Redlow, A.E.; Fick-Child, K.J.; Teas, W.G. Fibropapillomatosis in stranded green turtles (Chelonia mydas) from the eastern United States (1980-98): Trends and associations with environmental factors. J. Wildl. Dis. 2005, 41, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, R.G.; Martins, A.S.; Torezani, E.; Baptistotte, C.; Nobrega, F.J.; Horta, P.A.; Work, T.M.; Balazs, G.H. Relationship between fibropapillomatosis and environmental quality: A case study with Chelonia mydas off Brazil. Dis. Aquat. Organ. 2010, 89, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Lutz, P.L.; Cray, C.; Sposato, P.L. Studies of the Association between Immunosuppression and Fibropapillomatosis within Three Habitats of Chelonia mydas; NOAA Technical Memorandum Administrative Report H-01-01C; National Marine Fisheries Service, Scientific Publications Office: Seattle, WA, USA, 2001; p. 26. [Google Scholar]
- Cray, C.; Varella, R.; Bossart, G.D.; Lutz, P.L. Altered in vitro immune responses in green turtles (Chelonia mydas) with fibropapillomatosis. J. Zoo Wildl. Med. 2001, 32, 436–440. [Google Scholar]
- Aguirre, A.A.; Balazs, G.H.; Spraker, T.R.; Gross, T.S. Adrenal and hematological responses to stress in juvenile green turtles (Chelonia mydas) with and without fibropapillomas. Physiol. Zool. 1995, 68, 831–854. [Google Scholar] [CrossRef]
- Work, T.M.; Rameyer, R.A.; Balazs, G.H.; Cray, C.; Chang, S.P. Immune status of free-ranging green turtles with fibropapillomatosis from Hawaii. J. Wildl. Dis. 2001, 37, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Sposato, P.; Keating, P.; Lutz, P.L.; Milton, S.L. Evaluation of immune function in two populations of green sea turtles (Chelonia mydas) in a degraded versus a nondegraded habitat. J. Wildl. Dis. 2021, 57, 761–772. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Norton, T.M.; Krimer, P.; Groner, M.; Nelson, S.E.; Gottdenker, N.L. Factors influencing survivorship of rehabilitating green sea turtles (Chelonia mydas) with fibropapillomatosis. J. Zoo Wildl. Med. 2014, 45, 507–519. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Perrault, J.R.; Zirkelbach, B.; Pescatore, J.; Riley, R.; Stadler, M.; Zachariah, T.T.; Marks, W.; Norton, T.M. Tumor re-growth, case outcome, and tumor scoring systems in rehabilitated green turtles with fibropapillomatosis. Dis. Aquat. Organ. 2019, 137, 101–108. [Google Scholar] [CrossRef]
- Stacy, B.A.; Foley, A.M.; Work, T.M.; Lauritsen, A.M.; Schroeder, B.A.; Hargrove, S.K.; Keene, J.L. Report of the Technical Expert Workshop: Developing Recommendations for Field Response, Captive Management, and Rehabilitation of Sea Turtles with Fibropapillomatosis; NOAA Technical Memorandum NMFS OPR-60; U.S. Department of Commerce, National Marine Fisheries Service: St. Petersburg, FL, USA, 2018; 56p. [Google Scholar]
- Chao, C.T.; Chiang, C.K.; Huang, J.W.; Hung, K.Y. Vitamin D is closely linked to the clinical courses of herpes zoster: From pathogenesis to complications. Med. Hypotheses 2015, 85, 452–457. [Google Scholar] [CrossRef]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.E.; Brooks, H.L.; Boitano, S.; Barman, S.M. Ganong’s Review of Medical Physiology, 23rd ed.; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the immune system: Genomic and non-genomic actions. Mini Rev. Med. Chem. 2015, 15, 953. [Google Scholar] [CrossRef] [PubMed]
- Valrance, M.E.; Brunt, A.H.; Welsh, J. Vitamin D receptor-dependant inhibition of mammary tumor growth by EB1089 and ultraviolet radiation in vivo. Endocrinology 2007, 148, 4887–4894. [Google Scholar] [CrossRef]
- Watkins, R.R.; Lemonovich, T.L.; Salata, R.A. An update on the association of vitamin D deficiency with common infectious diseases. Can. J. Physiol. Pharmacol. 2015, 93, 363–368. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and immune function: An overview. Proc. Nutr. Soc. 2012, 71, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- De Smet, K.; Contreras, R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol. Lett. 2005, 27, 1337–1347. [Google Scholar] [CrossRef]
- Dioguardi, M.; Guardiola, F.A.; Vazzana, M.; Cuesta, A.; Esteban, M.A.; Cammarata, M. Vitamin D3 affects innate immune status of European sea bass (Dicentrarchus labrax L.). Fish Physiol. Biochem. 2017, 43, 1161–1174. [Google Scholar] [CrossRef]
- Estévez, R.A.; Mostazo, M.; Rodriguez, E.; Espinoza, J.C.; Kuznar, J.; Jónsson, Z.O.; Guðmundsson, G.H.; Maier, V.H. Inducers of salmon innate immunity: An in vitro and in vivo approach. Fish Shellfish Immunol. 2018, 72, 247–258. [Google Scholar] [CrossRef]
- Acierno, M.J.; Mitchell, M.A.; Zachariah, T.T.; Roundtree, M.K.; Kirchgessner, M.S.; Sanchez-Migallon Guzman, D. Effects of ultraviolet radiation on plasma 25-hydroxyvitamin D3 concentrations in corn snakes (Elaphe guttata). Am. J. Vet. Res. 2008, 69, 294–297. [Google Scholar] [CrossRef] [Green Version]
- Acierno, M.J.; Mitchell, M.A.; Roundtree, M.K.; Zachariah, T.T. Effects of ultraviolet radiation of 25-hydroxyvitamin D3 synthesis in red-eared slider turtles (Trachemys scripta elegans). Am. J. Vet. Res. 2007, 67, 2046–2049. [Google Scholar] [CrossRef]
- Laing, C.J.; Trube, A.; Shea, G.M.; Fraser, D.R. The requirement for natural sunlight to prevent vitamin D deficiency in iguanian lizards. J. Zoo Wildl. Med. 2001, 32, 342–348. [Google Scholar]
- Oonincx, D.G.A.B.; Stevens, Y.; Van den Borne, J.J.G.C.; Van Leeuwen, J.P.T.M.; Hendriks, W.H. Effects of vitamin D3 supplementation and UVb exposure on the growth and plasma concentration of vitamin D3 metabolites in juvenile bearded dragons (Pogona vitticeps). Comp. Biochem. Physiol. B 2010, 156, 122–128. [Google Scholar] [CrossRef]
- Purgley, H.; Jewell, J.; Deacon, J.E.; Winokur, R.M.; Tripoli, V.M. Vitamin D3 in Captive Green Sea turtles (Chelonia mydas). Chelonian Conserv. Biol. 2009, 8, 161–167. [Google Scholar] [CrossRef]
- Stringer, E.M.; Harms, C.A.; Beasley, J.F.; Anderson, E.T. Comparison of ionized calcium, parathyroid hormone, and 25-hydroxyvitamin D in rehabilitating and healthy wild green sea turtles (Chelonia mydas). J. Herpetol. Med. Surg. 2010, 20, 122–127. [Google Scholar] [CrossRef]
- Bloodgood, J.C.G.; Norton, T.M.; Hoopes, L.A.; Stacy, N.I.; Hernandez, S.M. Comparison of hematological, plasma biochemical, and nutritional analytes of rehabilitating and apparently healthy free-ranging Atlantic green turtles (Chelonia mydas). J. Zoo Wildl. Med. 2019, 50, 69–81. [Google Scholar]
- Lillywhite, H.B.; Smits, A.W. Lability of blood volume in snakes and its relation to activity and hypertension. J. Exp. Biol. 1984, 110, 267–274. [Google Scholar] [CrossRef]
- Smits, A.W.; Kozubowski, M.M. Partitioning of body fluids and cardiovascular responses to circulatory hypovolaemia in the turtle, Pseudemys scripta elegans. J. Exp. Biol. 1985, 116, 237–250. [Google Scholar] [CrossRef]
- Body Condition Scoring the Sea Turtle. Available online: https://lafeber.com/vet/body-condition-scoring-the-sea-turtle/ (accessed on 13 September 2021).
- Herbst, L.H. Fibropapillomatosis of marine turtles. Annu. Rev. Fish Dis. 1994, 4, 389–425. [Google Scholar] [CrossRef]
- Hirama, S.; Ehrhart, L.M. Description, prevalence and severity of green turtle fibropapillomatosis in three developmental habitats on the east coast of Florida. Fla. Sci. 2007, 70, 435–448. [Google Scholar]
- Zwarg, T.; Rossi, S.; Sanches, T.C.; Cesar, M.O.; Werneck, M.R.; Matushima, E.R. Hematological and histopathological evaluation of wildlife green turtles (Chelonia mydas) with and without fibropapilloma from the north coast of São Paulo State, Brazil. Pesq. Vet. Bras. 2014, 34, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Work, T.M.; Balazs, G.H. Relating tumor score to hematology in green turtles with fibropapillomatosis in Hawaii. J. Wildl. Dis. 1999, 35, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Hirama, S.; Ehrhart, L.M.; Rea, L.D.; Kiltie, R.A. Relating fibropapilloma tumor severity to blood parameters in green turtles Chelonia mydas. Dis. Aquat. Org. 2014, 111, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Owen, J.A.; Punt, J.; Strandford, S.A.; Jones, P.P. Kuby Immunology, 7th ed.; W.H. Freeman and Company: New York, New York, USA, 2013. [Google Scholar]
- Varela, R.A. The immunology of green turtle fibropapillomatosis. Master’s Thesis, Florida Atlantic University, Boca Raton, FA, USA, 1997. [Google Scholar]
- Aguirre, A.A.; Balazs, G.H. Blood biochemistry value of green turtles, Chelonia mydas, with and without fibropapillomatosis. Comp. Haematol. Int. 2000, 10, 132–137. [Google Scholar] [CrossRef]
- Lockau, L.; Atkinson, S.A. Vitamin D’s role in health and disease: How does the present inform our understanding of the past? Int. J. Paleopathol. 2018, 23, 6–14. [Google Scholar] [CrossRef]
- Jakobsen, J.; Jensen, S.K.; Hymøller, L.; Anderson, E.W.; Kaas, P.; Burild, A.; Jäpelt, R.B. Short communication: Artificial ultraviolet B light exposure increases vitamin D levels in cow plasma and milk. J. Dairy Sci. 2015, 98, 6492–6498. [Google Scholar] [CrossRef]
- Ferguson, G.W.; Gehrmann, W.H.; Hammack, S.H.; Chen, T.C.; Holick, M.F. Effects of dietary vitamin D and UV-B exposure on voluntary exposure to ultraviolet light, growth and survival of the panther chameleon. In Biologic Effects of Light 2001; Holick, M.F., Ed.; Springer: Boston, MA, USA, 2002; pp. 193–203. [Google Scholar]
- Karsten, K.B.; Ferguson, G.W.; Chen, T.C.; Holick, M.E. Panther chameleons, Furcifer pardalis, behaviorally regulate optimal exposure to UV depending on dietary vitamin D3 status. Physiol. Biochem. Zool. 2009, 82, 218–225. [Google Scholar] [CrossRef]
- Donoghue, S. Nutrition. Reptile Medicine and Surgery, 2nd ed.; Mader, D.R., Ed.; Saunders Elsevier: St. Louis, MI, USA, 2006; pp. 251–298. [Google Scholar]
- Flint, M.; Morton, J.M.; Limpus, C.J.; Patterson-Kane, J.C.; Murray, P.J.; Mills, P.C. Development and application of biochemical and haematological reference intervals to identify unhealthy green sea turtles (Chelonia mydas). Vet. J. 2010, 185, 299–304. [Google Scholar] [CrossRef]
- Nussey, S.; Whitehead, S. Endocrinology: An Integrated Approach; BIOS Scientific Publishers: Oxford, UK, 2001. [Google Scholar]
- Leary, P.F.; Zamfirova, I.; Au, J.; McCracken, W.H. Effect of Latitude on Vitamin D Levels. J. Am. Osteopath. Assoc. 2017, 117, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Southworth, L.O.; Holick, M.F.; Chen, T.C.; Kunz, T.H. Effects of sunlight on behavior and 25-hydroxyvitamin D levels in two species of old world fruit bats. Dermatoendocrinology 2013, 5, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Duffy, D.J.; Schnitzler, C.; Karpinski, L.; Thomas, R.; Whilde, J.; Eastman, C.; Yang, C.; Krstic, A.; Rollinson, D.; Zirkelbach, B.; et al. Sea turtle fibopapilloma tumors share genomic drivers and therapeutic vulnerabilities with human cancers. Commun. Biol. 2018, 1, 63. [Google Scholar] [CrossRef]
- Musick, J.A.; Limpus, C.J. Habitat utilization and migratino in juvenile sea turtles. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: Boca Raton, FA, USA, 1997; Volume 1, pp. 137–163. [Google Scholar]
- Häder, D.P.; Williamson, C.E.; Wängberg, S.Å.; Rautio, M.; Rose, K.C.; Gao, K.; Helbling, E.W.; Sinha, R.P.; Worrest, R. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochem. Photobiol. Sci. 2015, 14, 108–126. [Google Scholar] [CrossRef] [Green Version]
- Laing, C.J.; Fraser, D.R. The vitamin D system in iguanian lizards. Comp. Biochem. Physiol. B Biochem. 1999, 123, 373–379. [Google Scholar] [CrossRef]
- Perrault, J.R.; Levin, M.; Mott, C.R.; Bovery, C.M.; Bresette, M.J.; Chabot, R.M.; Gregory, C.R.; Guertin, J.R.; Hirsch, S.E.; Ritchie, B.W.; et al. Insights on immune function in free-ranging green sea turtles (Chelonia mydas) with and without fibropapillomatosis. Animals 2021, 11, 861. [Google Scholar] [CrossRef]
- Rousselet, E.; Stacy, N.I.; LaVictoire, K.; Higgins, B.M.; Tocidlowski, M.E.; Flanagan, J.P.; Godard-Codding, C.A.J. Hematology and plasma biochemistry analytes in five age groups of immature, captive-reared loggerhead sea turtles (Caretta caretta). J. Zoo Wildl. Med. 2013, 44, 859–874. [Google Scholar] [CrossRef]





| Chemistry Parameter | VT− Mean/ Median (n = 11) | VT− Range | VT+ Mean/ Median (n = 12) | VT+ Range | Significance |
|---|---|---|---|---|---|
| Glucose | 130 | 102–183 | 78.6 | 16–156 | p < 0.01 |
| BUN | 49 | 2–105 | 60.9 | 16–130 | n.s. |
| Uric Acid * | 0.9 | 0.5–4.5 | 1.5 | 0.4–3.3 | n.s. |
| Phosphorus | 7.1 | 4.7–11.8 | 7.4 | 2.7–10.5 | n.s. |
| Calcium * | 7.5 | 6.1–11.1 | 5.5 | 4.1–6.3 | p < 0.001 |
| Sodium | 155.7 | 148–161 | 153.7 | 149–159 | n.s. |
| Potassium | 4.2 | 2.9–4.7 | 4.3 | 3.4–5.2 | n.s. |
| Na:K Ratio * | 35.5 | 33–53 | 36 | 30–46 | n.s. |
| Chloride | 130.6 | 122–141 | 125.6 | 116–135 | n.s. |
| Total Protein | 4 | 1.9–5 | 3.4 | 2.3–4.6 | n.s. |
| Albumin | 1.4 | 0.8–2 | 1.1 | 0.5–1.5 | p < 0.05 |
| Globulin | 2.6 | 1.1–3.6 | 2.2 | 1.5–3.1 | n.s. |
| Albumin:Globulin Ratio | 0.55 | 0.4–0.7 | 0.53 | 0.3–0.8 | n.s. |
| ALT * | 14 | 10–102 | 11 | 2–212 | n.s. |
| ALP * | 23 | 10–52 | 17.5 | 10–52 | n.s. |
| GGT * | 0 | 0–3 | 0 | 0–1 | n.s. |
| Bilirubin * | 0.2 | 0.1–0.4 | 0.1 | 0.1–0.5 | n.s. |
| Cholesterol * | 85.5 | 55–152 | 9 | 6–119 | p < 0.001 |
| Amylase | 396 | 178–696 | 333 | 77–723 | n.s. |
| Lipase * | 13.5 | 10–33 | 21 | 10–88 | n.s. |
| Osmolality | 322.5 | 292–339 | 312.3 | 300–328 | n.s. |
| Group | Intake | <30 Days in Rehab | 30–60 Days in Rehab | 60–90 Days in Rehab | 90–120 Days in Rehab | 120–150 Days in Rehab | 150–180 Days in Rehab |
|---|---|---|---|---|---|---|---|
| VT−UV− | 5 | 2 | 3 | 1 | 0 | 0 | 0 |
| VT−UV+ | 6 | 1 | 4 | 1 | 0 | 0 | 0 |
| VT+UV− | 7 | 0 | 1 | 3 | 2 | 1 | 0 |
| VT+UV+ | 5 | 0 | 2 | 4 | 2 | 3 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garefino, V.E.; Milton, S.L. Influence of Sunlight on Vitamin D and Health Status in Green (Chelonia mydas) Sea Turtles with Fibropapillomatosis. Animals 2022, 12, 488. https://doi.org/10.3390/ani12040488
Garefino VE, Milton SL. Influence of Sunlight on Vitamin D and Health Status in Green (Chelonia mydas) Sea Turtles with Fibropapillomatosis. Animals. 2022; 12(4):488. https://doi.org/10.3390/ani12040488
Chicago/Turabian StyleGarefino, Victoria E., and Sarah L. Milton. 2022. "Influence of Sunlight on Vitamin D and Health Status in Green (Chelonia mydas) Sea Turtles with Fibropapillomatosis" Animals 12, no. 4: 488. https://doi.org/10.3390/ani12040488
APA StyleGarefino, V. E., & Milton, S. L. (2022). Influence of Sunlight on Vitamin D and Health Status in Green (Chelonia mydas) Sea Turtles with Fibropapillomatosis. Animals, 12(4), 488. https://doi.org/10.3390/ani12040488