Porcine Germ Cells Phenotype during Embryonic and Adult Development
Abstract
:Simple Summary
Abstract
1. Introduction
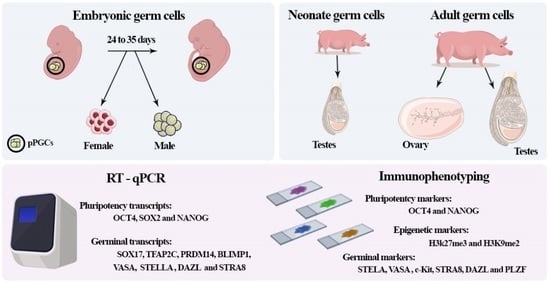
2. Materials and Methods
2.1. Sample Collection
2.2. Sexing of Collected Embryos
2.3. Histological Analysis
2.4. Immunofluorescence Analysis
2.5. qRT-PCR
2.6. Statistical Analysis
3. Results
3.1. Morphological and Immunofluorescence Analysis
3.1.1. Male Gonads
3.1.2. Female Gonads
3.2. Analysis of the Gene Expression in Porcine Germ Cells
4. Discussion
4.1. Histological and Immunophenotype Analysis of the Porcine Germ Cells
4.2. Epigenetic Markers in Porcine Germ Cells
4.3. Analysis of the Gene Expression in Porcine Germ Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, L.J.; LeBlanc, M.G.; Lehmann, R. Finding their way: Themes in germ cell migration. Curr. Opin. Cell Biol. 2016, 42, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Kanamori, M.; Oikawa, K.; Tanemura, K.; Hara, K. Mammalian germ cell migration during development, growth, and homeostasis. Reprod. Med. Biol. 2019, 18, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyttel, P.; Sinowatz, F.; Vejlsted, M.; Betteridge, K.J. Embriologia Veterinária; Elsevier Editora Ltda.: São Paulo, Brazil, 2012; ISBN 9788535265187. [Google Scholar]
- Kobayashi, T.; Zhang, H.; Tang, W.W.C.C.; Irie, N.; Withey, S.; Klisch, D.; Sybirna, A.; Dietmann, S.; Contreras, D.A.; Webb, R.; et al. Principles of early human development and germ cell program from conserved model systems. Nature 2017, 546, 416–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, P.P.L.; Zhou, S.X. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev. Biol. 1996, 178, 124–132. [Google Scholar] [CrossRef]
- Lawson, K.A.; Dunn, N.R.; Roelen, B.A.; Zeinstra, L.M.; Davis, A.M.; Wright, C.V.; Korving, J.P.; Hogan, B.L. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999, 13, 424–436. [Google Scholar] [CrossRef]
- Golkar-Narenji, A.; Dziegiel, P.; Kempisty, B.; Petitte, J.; Mozdziak, P.E.; Bryja, A. In vitro culture of reptile PGCS to preserve endangered species. Cell Biol. Int. 2023, 47, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Hancock, G.V.; Wamaitha, S.E.; Peretz, L.; Clark, A.T. Mammalian primordial germ cell specification. Development 2021, 148, dev189217. [Google Scholar] [CrossRef]
- Nakaki, F.; Hayashi, K.; Ohta, H.; Kurimoto, K.; Yabuta, Y.; Saitou, M. Induction of mouse germ-cell fate by transcription factors in vitro. Nature 2013, 501, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Magnúsdóttir, E.; Dietmann, S.; Murakami, K.; Günesdogan, U.; Tang, F.; Bao, S.; Diamanti, E.; Lao, K.; Gottgens, B.; Surani, M.A. A tripartite transcription factor network regulates primordial germ cell specification in mice. Nat. Cell Biol. 2013, 15, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Irie, N.; Weinberger, L.; Tang, W.W.C.C.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Shang, D.; Zhou, R. Germline stem cells in human. Signal Transduct. Target. Ther. 2022, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Payer, B.; Saitou, M.; Barton, S.C.; Thresher, R.; Dixon, J.P.C.; Zahn, D.; Colledge, W.H.; Carlton, M.B.L.; Nakano, T.; Surani, M.A. stella Is a Maternal Effect Gene Required for Normal Early Development in Mice. Curr. Biol. 2003, 13, 2110–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.; Lee, W.Y.; Park, H.J.; Ha, W.T.; Woo, J.S.; Chung, H.J.; Lee, J.H.; Hong, K.; Song, H. Stage-specific expression of DDX4 and c-kit at different developmental stages of the porcine testis. Anim. Reprod. Sci. 2018, 190, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Castrillon, D.H.; Quade, B.J.; Wang, T.Y.; Quigley, C.; Crum, C.P. The human VASA gene is specifically expressed in the germ cell lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 9585–9590. [Google Scholar] [CrossRef] [PubMed]
- Abdyyev, V.K.; Dashinimayev, E.B.; Neklyudova, I.V.; Vorotelyak, E.A.; Vasiliev, A. V Modern Technologies Deriving Human Primordial Germ Cells in vitro. Biochemistry 2019, 84, 220–231. [Google Scholar] [CrossRef]
- Hyldig, S.M.W.; Croxall, N.; Contreras, D.A.; Thomsen, P.D.; Alberio, R. Epigenetic reprogramming in the porcine germ line. BMC Dev. Biol. 2011, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Pilato, G.; D’Urso, V.; Viglianisi, F.; Sammartano, F.; Sabella, G.; Lisi, O. The problem of the origin of primordial germ cells (PGCs) in vertebrates: Historical review and a possible solution. Int. J. Dev. Biol. 2013, 57, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, P.K.; Schorle, H.; Naqvi, S.; Hu, Y.C.; Fan, Y.; Carmell, M.A.; Dobrinski, I.; Watson, A.L.; Carlson, D.F.; Fahrenkrug, S.C.; et al. Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc. Natl. Acad. Sci. USA 2019, 116, 25677–25687. [Google Scholar] [CrossRef]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Chu, Y.; Zhu, H.; Sun, J.; Pu, Y.; Gao, Z.; Yang, C.; Peng, S.; Dou, Z.; Hua, J. Characterization of immortalized mesenchymal stem cells derived from foetal porcine pancreas. Cell Prolif. 2011, 44, 19–32. [Google Scholar] [CrossRef]
- Lv, X.; Zhu, H.; Bai, Y.; Chu, Z.; Hu, Y.; Cao, H.; Liu, C.; He, X.; Peng, S.; Gao, Z.; et al. Reversine promotes porcine muscle derived stem cells (PMDSCs) differentiation into female germ-like cells. J. Cell. Biochem. 2012, 113, 3629–3642. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Wu, S.; Wu, C.; Li, N.; Zhu, H.; Liu, W. Multipotent male germline stem cells (mGSCs) from neonate porcine testis. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Parma, P.; Pailhoux, E.; Cotinot, C. Reverse Transcription-Polymerase Chain Reaction Analysis of Genes Involved in Gonadal Differentiation in Pigs. Biol. Reprod. 1999, 61, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X. Investigating the Genetic Program of Germ Cell Specification in Pig; University of Nottingham: Nottingham, UK, 2010. [Google Scholar]
- Wang, H.; Xiang, J.; Zhang, W.; Li, J.; Wei, Q.; Zhong, L.; Ouyang, H.; Han, J. Induction of Germ Cell-like Cells from Porcine Induced Pluripotent Stem Cells. Sci. Rep. 2016, 6, 27256. [Google Scholar] [CrossRef] [Green Version]
- Pieri, N.C.G.; de Souza, A.F.; Botigelli, R.C.; de Figueiredo Pessôa, L.V.; Recchia, K.; Machado, L.S.; Glória, M.H.; de Castro, R.V.G.; Leal, D.F.; Fantinato Neto, P.; et al. Porcine Primordial Germ Cell-Like Cells Generated from Induced Pluripotent Stem Cells Under Different Culture Conditions. Stem Cell Rev. Rep. 2021, 18, 1639–1656. [Google Scholar] [CrossRef]
- Petkov, S.G.; Reh, W.A.; Anderson, G.B. Methylation changes in porcine primordial germ cells. Mol. Reprod. Dev. 2009, 76, 22–30. [Google Scholar] [CrossRef]
- Hyldig, M.S.; Watjen, O.; Olga, V.; Morten, T.; Preben, D. Changes of DNA Methylation Level and Spatial Arrangement of Primordial Germ Cells in Embryonic Day 15 to Embryonic Day 28 Pig Embryos. Biol. Reprod. 2011, 84, 1087–1093. [Google Scholar] [CrossRef] [Green Version]
- Aponte, P.M.; Aponte, P.M.; Prometeo, A.P. Spermatogonial stem cells: Current biotechnological advances in reproduction and regenerative medicine. World J. Stem Cells 2015, 7, 669. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Míguez-Forjan, J.M.; Simón, C. Artificial gametes from stem cells. Clin. Exp. Reprod. Med. 2015, 42, 33. [Google Scholar] [CrossRef] [Green Version]
- Pieri, N.C.G.N.C.G.; de Souza, A.F.A.F.; Botigelli, R.C.R.C.; Machado, L.S.L.S.; Ambrosio, C.E.C.E.; dos Santos Martins, D.; de Andrade, A.F.C.A.F.C.; Meirelles, F.V.F.V.; Hyttel, P.; Bressan, F.F.F.F. Stem cells on regenerative and reproductive science in domestic animals. Vet. Res. Commun. 2019, 43, 7–16. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Khoirinaya, C.; Kim, S.J.; Moon, J.H.; Almadaly, E.; Lee, B.C. Blastocysts derivation from somatic cell fusion with premature oocytes (prematuration somatic cell fusion). Dev. Growth Differ. 2016, 58, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Aasen, E.; Medrano, J.F. Amplification of the ZFY and ZFX genes for sex identification in humans, cattle, sheep and goats. Bio/Technology 1990, 8, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Pomp, D.; Good, B.A.; Geisert, R.D.; Corbin, C.J.; Conley, A.J. Sex identification in mammals with polymerase chain reaction and its use to examine sex effects on diameter of day-10 or -11 pig embryos. J. Anim. Sci. 1995, 73, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Pieri, N.C.G.; Souza, A.F.; Casals, J.B.; Roballo, K.C.S.; Ambrósio, C.E.; Martins, D.S. Comparative Development of Embryonic Age by Organogenesis in Domestic Dogs and Cats. Reprod. Domest. Anim. 2015, 50, 625–631. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.F.A.F.; de Ramos, E.C.E.C.; Cury, F.S.F.S.; Pieri, N.C.G.N.C.G.; Martins, D.S.D.S. The timeline development of female canine germ cells. Reprod. Domest. Anim. 2019, 54, 964–971. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.F.A.F.; Godoy Pieri, N.C.N.C.; Roballo, K.C.S.K.C.S.; Bressan, F.F.F.; Casals, J.B.J.B.; Ambrósio, C.E.C.E.; Perecin, F.; Martins, D.S.D.S. Dynamics of male canine germ cell development. PLoS ONE 2018, 13, e0193026. [Google Scholar] [CrossRef] [Green Version]
- Machado, L.S.; Recchia, K.; Pieri, N.C.G.; Botigelli, R.C.; de Castro, R.V.G.; Brunhara Cruz, J.; de Figueiredo Pessôa, L.V.; Bressan, F.F. Differentiation of Porcine Induced Pluripotent Stem Cells (piPSCs) into Neural Progenitor Cells (NPCs). J. Vis. Exp. 2021, 172, e62209. [Google Scholar] [CrossRef]
- Anderson, R.A.; Fulton, N.; Cowan, G.; Coutts, S.; Saunders, P.T.K. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev. Biol. 2007, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Nef, S.; Stévant, I.; Greenfield, A. Characterizing the bipotential mammalian gonad. Curr. Top. Dev. Biol. 2019, 134, 167–194. [Google Scholar] [CrossRef]
- Ross, D.G.F.; Bowles, J.; Hope, M.; Lehnert, S.; Koopman, P. Profiles of Gonadal Gene Expression in the Developing Bovine Embryo. Sex. Dev. 2009, 3, 273–283. [Google Scholar] [CrossRef]
- Lee, C.-K.; Piedrahita, J.A. Effects of growth factors and feeder cells on porcine primordial germ cells in vitro. Cloning 2000, 2, 197–205. [Google Scholar] [CrossRef]
- Saitou, M.; Miyauchi, H. Gametogenesis from Pluripotent Stem Cells. Cell Stem Cell 2016, 18, 721–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, M.; Yamaji, M. Primordial germ cells in mice. Cold Spring Harb. Perspect. Biol. 2012, 4, a008375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehler, J.; Tolkunova, E.; Koschorz, B.; Pesce, M.; Gentile, L.; Boiani, M.; Nagy, A.; Mclaughlin, K.J.; Scho, H.R.; Lomelı, H. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004, 5, 1078–1083. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.S.; Kim, H.S.; Lee, S.H.; Kang, M.S.; Kim, D.Y.; Lee, C.K.; Kang, S.K.; Lee, B.C.; Hwang, W.S. Characterization of pig vasa homolog gene and specific expression in germ cell lineage. Mol. Reprod. Dev. 2005, 72, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Page, D.C. Dazl deficiency leads to embryonic arrest of germ cell development in XY C57BL/6 mice. Dev. Biol. 2005, 288, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrans-Stassen, B.H.; Saunders, P.T.; Cooke, H.J.; de Rooij, D.G. Nature of the spermatogenic arrest in Dazl -/- mice. Biol. Reprod. 2001, 65, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Welling, M.; Bloch, D.B.; Muñoz, J.; Mientjes, E.; Chen, X.; Tramp, C.; Wu, J.; Yabuuchi, A.; Chou, Y.F.; et al. DAZL limits pluripotency, differentiation, and apoptosis in developing primordial germ cells. Stem Cell Rep. 2014, 35, 892–904. [Google Scholar] [CrossRef] [Green Version]
- Gill, M.E.; Hu, Y.C.; Lin, Y.; Page, D.C. Licensing of gametogenesis, dependent on RNA binding protein DAZL, as a gateway to sexual differentiation of fetal germ cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7443–7448. [Google Scholar] [CrossRef]
- Savvulidi, F.; Ptacek, M.; Savvulidi Vargova, K.; Stadnik, L. Manipulation of spermatogonial stem cells in livestock species. J. Anim. Sci. Biotechnol. 2019, 10, 46. [Google Scholar] [CrossRef]
- Clotaire, D.Z.J.; Wei, Y.; Yu, X.; Ousman, T.; Hua, J. Functions of promyelocytic leukaemia zinc finger (Plzf) in male germline stem cell development and differentiation. Reprod. Fertil. Dev. 2019, 31, 1315–1320. [Google Scholar] [CrossRef]
- Goel, S.; Sugimoto, M.; Minami, N.; Yamada, M.; Kume, S.; Imai, H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol. Reprod. 2007, 77, 127–137. [Google Scholar] [CrossRef]
- Goel, S.; Fujihara, M.; Minami, N.; Yamada, M.; Imai, H. Expression of NANOG, but not POU5F1, points to the stem cell potential of primitive germ cells in neonatal pig testis. Reproduction 2008, 135, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Niedenberger, B.A.; Busada, J.T.; Geyer, C.B. Marker expression reveals heterogeneity of spermatogonia in the neonatal mouse testis. Reproduction 2015, 149, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Menke, D.B.; Koubova, J.; Page, D.C. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev. Biol. 2003, 262, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.L.; Baltus, A.E.; Roepers-Gajadien, H.L.; Hassold, T.J.; De Rooij, D.G.; Van Pelt, A.M.M.; Page, D.C. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 14976–14980. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Nie, R.; Li, Y.; Friel, P.; Mitchell, D.; Hess, R.A.; Small, C.; Griswold, M.D. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: An in vivo study in vitamin A-sufficient postnatal murine testes. Biol. Reprod. 2008, 79, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harkey, M.A.; Asano, A.; Zoulas, M.E.; Torok-Storb, B.; Nagashima, J.; Travis, A. Isolation, genetic manipulation, and transplantation of canine spermatogonial stem cells: Progress toward transgenesis through the male germ-line. Reproduction 2013, 146, 75–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Lee, R.; Lee, W.Y.; Kim, D.H.; Chung, H.J.; Kim, J.H.; Kim, N.H.; Choi, S.H.; Kim, J.H.; Song, H. Identification and in vitro derivation of spermatogonia in beagle testis. PLoS ONE 2014, 9, e109963. [Google Scholar] [CrossRef]
- Pieri, N.C.G.N.C.G.; Souza, A.F.F.A.; Mançanares, A.C.F.C.F.; Roballo, K.K.C.S.C.S.; Casals, J.B.J.B.; Ambrosio, C.C.E.E.; Martins, D.S.D.S.; Man??anares, A.; Roballo, K.K.C.S.C.S.; Casals, J.B.J.B.; et al. Immunolocalization of proteins in the spermatogenesis process of canine. Reprod. Domest. Anim. 2016, 52, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, F.; Zhang, L.; Lei, P.; Zheng, Y.; Zeng, W. Stage-specific embryonic antigen 4 is a membrane marker for enrichment of porcine spermatogonial stem cells. Andrology 2020, 8, 1923–1934. [Google Scholar] [CrossRef]
- Schrans-Stassen, B.H.G.J.; Van De Kant, H.J.G.; De Rooij, D.G.; Van Pelt, A.M.M. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 1999, 140, 5894–5900. [Google Scholar] [CrossRef]
- Evans, A.C.O. Characteristics of ovarian follicle development in domestic animals. Reprod. Domest. Anim. 2003, 38, 240–246. [Google Scholar] [CrossRef]
- Brekhman, V.; Itskovitz-Eldor, J.; Yodko, E.; Deutsch, M.; Seligman, J. The DAZL1 gene is expressed in human male and female embryonic gonads before meiosis. Mol. Hum. Reprod. 2000, 6, 465–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauffman, G.; Van de Velde, H.; Liebaers, I.; Van Steirteghem, A. DAZL expression in human oocytes, preimplantation embryos and embryonic stem cells. Mol. Hum. Reprod. 2005, 11, 405–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorfman, D.M.; Genest, D.R.; Reijo Fera, R.A. Human DAZL1 encodes a candidate fertility factor in women that localizes to the prenatal and postnatal germ cells. Hum. Reprod. 1999, 14, 2531–2536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.Y.; Chang, S.Y.; Lo, H.Y.; Chen, I.H.; Huang, F.J.; Kung, F.T.; Lu, Y.J. The expression of DAZL1 in the ovary of the human female fetus. Fertil. Steril. 2000, 73, 627–630. [Google Scholar] [CrossRef]
- Liu, Y.J.; Nakamura, T.; Nakano, T. Essential role of DPPA3 for chromatin condensation in mouse oocytogenesis. Biol. Reprod. 2012, 86, 40–41. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Zhao, X.; Tian, G.G.; Wu, J. Stella Regulates the Development of Female Germline Stem Cells by Modulating Chromatin Structure and DNA Methylation. Int. J. Biol. Sci. 2022, 18, 3006. [Google Scholar] [CrossRef]
- Sato, M.; Kimura, T.; Kurokawa, K.; Fujita, Y.; Abe, K.; Masuhara, M.; Yasunaga, T.; Ryo, A.; Yamamoto, M.; Nakano, T. Identification of PGC7, a new gene expressed specifically in preimplantation embryos and germ cells. Mech. Dev. 2002, 113, 91–94. [Google Scholar] [CrossRef]
- Ohinata, Y.; Payer, B.; O’Carroll, D.D.; Ancelin, K.; Ono, Y.; Sano, M.; Barton, S.C.; Obukhanych, T.; Nussenzweig, M.; Tarakhovsky, A.; et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 2005, 436, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sekl, Y.; Yamaji, M.; Yabuta, Y.; Sano, M.; Shigeta, M.; Matsui, Y.; Saga, Y.; Tachibana, M.; Shinkai, Y.; Saitou, M.; et al. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development 2007, 134, 2627–2638. [Google Scholar] [CrossRef] [Green Version]
- Hackett, J.A.; Zylicz, J.J.; Surani, M.A. Parallel mechanisms of epigenetic reprogramming in the germline. Trends Genet. 2012, 28, 164–174. [Google Scholar] [CrossRef]
- Dean, W.; Santos, F.; Reik, W. Epigenetic reprogramming in early mammalian development and following somatic nuclear transfer. Semin. Cell Dev. Biol. 2003, 14, 93–100. [Google Scholar] [CrossRef]
- Surani, M.A.; Hayashi, K.; Hajkova, P. Genetic and Epigenetic Regulators of Pluripotency. Cell 2007, 128, 747–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, Y.; Hayashi, K.; Itoh, K.; Mizugaki, M. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev. Biol. 2005, 278, 440–458. [Google Scholar] [CrossRef] [Green Version]
- Chuva De Sousa Lopes, S.M.; Hayashi, K.; Shovlin, T.C.; Mifsud, W.; Surani, M.A.; McLaren, A. X Chromosome Activity in Mouse XX Primordial Germ Cells. PLoS Genet. 2008, 4, e30. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Sang, F.; Withey, S.; Tang, W.; Dietmann, S.; Klisch, D.; Ramos-Ibeas, P.; Zhang, H.; Requena, C.E.; Hajkova, P.; et al. Specification and epigenomic resetting of the pig germline exhibit conservation with the human lineage. Cell Rep. 2021, 34, 108735. [Google Scholar] [CrossRef]
- An, J.; Qin, J.; Wan, Y.; Zhang, Y.; Hu, Y.; Zhang, C.; Zeng, W. Histone lysine methylation exhibits a distinct distribution during spermatogenesis in pigs. Theriogenology 2015, 84, 1455–1462. [Google Scholar] [CrossRef]
- Brykczynska, U.; Hisano, M.; Erkek, S.; Ramos, L.; Oakeley, E.J.; Roloff, T.C.; Beisel, C.; Schübeler, D.; Stadler, M.B.; Peters, A.H.F.M. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 2010, 17, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, F.; Xu, D.; Lu, L.; Chen, Q.; Yang, Z.; Wang, X.; Pan, X. Wenshen Shengjing Decoction Improves Early Embryo Development by Maintaining Low H3K27me3 Levels in Sperm and Pronuclear Embryos of Spermatogenesis Impaired Mice. Evid. Based Complement. Alternat. Med. 2021, 2021, 8035997. [Google Scholar] [CrossRef] [PubMed]
- La Spina, F.A.; Romanato, M.; Brugo-Olmedo, S.; De Vincentiis, S.; Julianelli, V.; Rivera, R.M.; Buffone, M.G. Heterogeneous distribution of histone methylation in mature human sperm. J. Assist. Reprod. Genet. 2014, 31, 45. [Google Scholar] [CrossRef] [Green Version]
- Ewen, K.A.; Koopman, P. Mouse germ cell development: From specification to sex determination. Mol. Cell. Endocrinol. 2010, 323, 76–93. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Jie, H.Y.; Huang, K.J.; Cai, B.; Zhou, X.; Liang, M.Y.; Zhou, C.Q.; Mai, Q.Y. The Dynamic Expression of SOX17 in Germ Cells from Human Female Foetus and Adult Genital Glands after specification. Front. Endocrinol. 2023, 14, 1124143. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dumitriu, B.; Penzo-Méndez, A.; Han, Y.; Pallavi, B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int. J. Biochem. Cell Biol. 2007, 39, 2195–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, K.; Sarkar, A.; Yram, M.A.; Polo, J.M.; Bronson, R.; Sengupta, S.; Seandel, M.; Geijsen, N.; Hochedlinger, K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011, 9, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef]
- Bahmanpour, S.; Khozani, T.T.; Fard, N.Z.; Jaberipour, M.; Hosseini, A.; Esmaeilpour, T. A comparison of the multiple oocyte maturation gene expression patterns between the newborn and adult mouse ovary. Iran. J. Reprod. Med. 2013, 11, 815. [Google Scholar]
- Hansen, C.L.; Pelegri, F. Primordial Germ Cell Specification in Vertebrate Embryos: Phylogenetic Distribution and Conserved Molecular Features of Preformation and Induction. Front. Cell Dev. Biol. 2021, 9, 730332. [Google Scholar] [CrossRef]
- Xu, E.Y.; Moore, F.L.; Reijo Pera, R.A. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc. Natl. Acad. Sci. USA 2001, 98, 7414–7419. [Google Scholar] [CrossRef] [PubMed]
- Niikura, Y.; Niikura, T.; Wang, N.; Satirapod, C.; Tilly, J.L. Systemic signals in aged males exert potent rejuvenating effects on the ovarian follicle reserve in mammalian females. Aging 2010, 2, 999–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Satirapod, C.; Ohguchi, Y.; Park, E.S.; Woods, D.C.; Tilly, J.L. Genetic studies in mice directly link oocytes produced during adulthood to ovarian function and natural fertility. Sci. Rep. 2017, 7, 10011. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Li, C.H.; Wang, X.Y.; He, D.J.; Zheng, P. Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol. Hum. Reprod. 2016, 22, 316. [Google Scholar] [CrossRef] [Green Version]
- Imudia, A.N.; Wang, N.; Tanaka, Y.; White, Y.A.R.; Woods, D.C.; Tilly, J.L. Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity. Fertil. Steril. 2013, 100, 1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sequence Name | Sequence | Size (bp) | Gene Bank |
|---|---|---|---|
| OCT4 (POU5F1) | GGGTTCTCTTTGGGAAGGTGT CTCCAGGTTGCCTCTCACTC | 224 | NM_001113060.1 |
| SOX2 | CTCAGTGGTCAAGTCCGAGG AGAGAGAGGCAGTGTACCGT | 223 | NM_001123197.1 |
| NANOG | TCCTTCCTCCATGGATCTGCT GGGTCTGCGAGAACACAGTT | 155 | NM_001129971.1 |
| SOX17 | TCCACTCTGCTAGTGCCTCT CTGGGGATGCCCTAATGTTCA | 193 | XM_001928376.7 |
| TFAP2C | GAAACCCTGGACTGGACGAG GTAGCACCACTTGCAGAGGA | 121 | NM_001123201 |
| PRDM14 | AGTGGATGCTTCTCTGCTACC TGCCTTTCTCTCTTGGTTCA | 112 | [26] |
| BLIMP1 (PRDM1) | CAGTGCCGTGAAGTTTCC AAGGATGCCTCTGCCTGAAC | 186 | [26] |
| VASA (DDX4) | CCTGCCCAGGAATGCCATCA ACTGGCCAACTTGGAGAATGGT | 180 | [26] |
| STELLA (Dapp3) | CCCGCCTTTCAATCTGTCTCC TCGCCGAACCGTGTATCGAA | 219 | [26] |
| DAZL | GGTCGCTTTGCTTATCCGC TGCAGCAGACATTACTGCGA | 158 | [26] |
| STRA8 | TGGAGAAGGGAGCAACCCCA ACCTGCCACTTTGAGGCTGT | 189 | [26] |
| β-ACTIN | GAAGATCAAGATCATCGCGCCT GTGGAATGCAACTAACAGTCCG | 117 | XM_003124280.4 |
| GAPDH | GTCGGTTGTGGATCTGACCT ACCAGGAAATGAGCTTGACGA | 221 | NM_001206359.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, A.S.; Recchia, K.; Glória, M.H.; de Souza, A.F.; Pessôa, L.V.d.F.; Fantinato Neto, P.; Martins, D.d.S.; de Andrade, A.F.C.; Martins, S.M.M.K.; Bressan, F.F.; et al. Porcine Germ Cells Phenotype during Embryonic and Adult Development. Animals 2023, 13, 2520. https://doi.org/10.3390/ani13152520
Jorge AS, Recchia K, Glória MH, de Souza AF, Pessôa LVdF, Fantinato Neto P, Martins DdS, de Andrade AFC, Martins SMMK, Bressan FF, et al. Porcine Germ Cells Phenotype during Embryonic and Adult Development. Animals. 2023; 13(15):2520. https://doi.org/10.3390/ani13152520
Chicago/Turabian StyleJorge, Amanda Soares, Kaiana Recchia, Mayra Hirakawa Glória, Aline Fernanda de Souza, Laís Vicari de Figueirêdo Pessôa, Paulo Fantinato Neto, Daniele dos Santos Martins, André Furugen Cesar de Andrade, Simone Maria Massami Kitamura Martins, Fabiana Fernandes Bressan, and et al. 2023. "Porcine Germ Cells Phenotype during Embryonic and Adult Development" Animals 13, no. 15: 2520. https://doi.org/10.3390/ani13152520
APA StyleJorge, A. S., Recchia, K., Glória, M. H., de Souza, A. F., Pessôa, L. V. d. F., Fantinato Neto, P., Martins, D. d. S., de Andrade, A. F. C., Martins, S. M. M. K., Bressan, F. F., & Pieri, N. C. G. (2023). Porcine Germ Cells Phenotype during Embryonic and Adult Development. Animals, 13(15), 2520. https://doi.org/10.3390/ani13152520