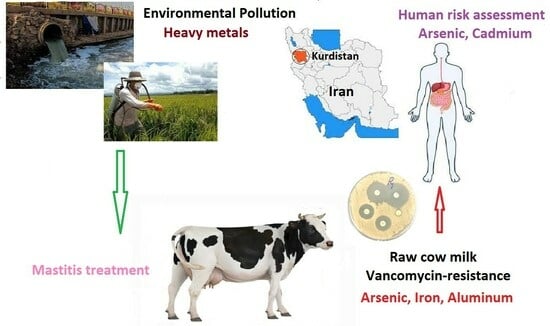
The Impact of Metal and Heavy Metal Concentrations on Vancomycin Resistance in Staphylococcus aureus within Milk Produced by Cattle Farms and the Health Risk Assessment in Kurdistan Province, Iran
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Sampling
2.2. Isolate and Identify Staphylococcus aureus
2.3. Biochemical Tests for the Diagnosis of Staphylococcus aureus
2.4. ICP-MS Method Determination
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grout, L.; Baker, M.G.; French, N.; Hales, S. A review of potential public health impacts associated with the global dairy sector. GeoHealth 2020, 4, e2019GH000213. [Google Scholar] [CrossRef] [PubMed]
- Krömker, V.; Leimbach, S. Mastitis treatment—Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. 2017, 52, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Barzoki, H.R.; Faraji, H.; Beirami, S.; Keramati, F.Z.; Nayik, G.A.; Yazdanaabadi, Z.I.; Nejad, A.S.M. Seasonal Study of Aflatoxin M1 Contamination in Cow Milk on the Retail Dairy Market in Gorgan. Iran. Dairy 2023, 4, 571–580. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef]
- Ismail, A.; Riaz, M.; Akhtar, S.; Goodwill, J.E.; Sun, J. Heavy metals in milk: Global prevalence and health risk assessment. Toxin Rev. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog? IntechOpen: London, UK, 2019; Volume 10, pp. 70–90. [Google Scholar]
- Haidar, Z.; Fatema, K.; Shoily, S.S.; Sajib, A.A. Disease-associated metabolic pathways affected by heavy metals and metalloid. Toxicol. Rep. 2023, 10, 554–570. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Gravandi, H.; Akbari, A. Determination of some heavy metals levels in the meat of animal species (sheep, beef, turkey, and ostrich) and carcinogenic health risk assessment in Kurdistan province in the west of Iran. Environ. Sci. Pollut. Res. 2022, 29, 62248–62258. [Google Scholar] [CrossRef]
- Pal, M.; Shuramo, M.Y.; Tewari, A.; Srivastava, J.P.; Steinmetz, C.H. Staphylococcus aureus from a Commensal to Zoonotic Pathogen: A Critical Appraisal. Int. J. Clin. Exp. Med. Res. 2023, 7, 220–228. [Google Scholar] [CrossRef]
- Beheshtipour, J.; Raeeszadeh, M. Evaluation of interleukin-10 and pro-inflammatory cytokine profile in calves naturally infected with neonatal calf diarrhea syndrome. Arch. Razi Inst. 2020, 75, 213. [Google Scholar]
- Milani, M.; Curia, R.; Shevlyagina, N.V.; Tatti, F. Staphylococcus aureus, in Bacterial Degradation of Organic and Inorganic Materials: Staphylococcus aureus Meets the Nanoworld; Springer: Berlin/Heidelberg, Germany, 2023; pp. 3–20. [Google Scholar]
- Fu, Y.; Zhu, Y.; Dong, H.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Effects of heavy metals and antibiotics on antibiotic resistance genes and microbial communities in soil. Process Saf. Environ. Prot. 2023, 169, 418–427. [Google Scholar] [CrossRef]
- Ejaz, H.; Junaid, K.; Yasmeen, H.; Naseer, A.; Alam, H.; Younas, S.; Qamar, M.U.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmad, N.; et al. Multiple antimicrobial resistance and heavy metal tolerance of biofilm-producing bacteria isolated from dairy and non-dairy food products. Foods 2022, 11, 2728. [Google Scholar] [CrossRef] [PubMed]
- Dweba, C.C.; Zishiri, O.T.; and El Zowalaty, M.E. Isolation and molecular identification of virulence, antimicrobial and heavy metal resistance genes in livestock-associated methicillin-resistant Staphylococcus aureus. Pathogens 2019, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Hussey, M.A. Gram stain protocols. Am. Soc. Microbiol. 2005, 1, 113–144. [Google Scholar] [CrossRef]
- Procop, G.W.; Church, D.L.; Hall, G.S.; Janda, W.M. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology; Jones & Bartlett Learning: Burlington, MA, USA, 2020. [Google Scholar]
- Dezfulian, A.; Salehian, M.T.; Amini, V.; Dabiri, H.; Azimirad, M.; Aslani, M.M.; Zali, M.R.; Fazel, I. Catalase-negative Staphylococcus aureus isolated from a diabetic foot ulcer. Iran. J. Microbiol. 2010, 2, 165. [Google Scholar]
- Wayne, P. Performance Standards for Antimicrobial Susceptibility Testing; Ninth Informational Supplement; CLSI: Berwyn, PA, USA, 2008. [Google Scholar]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Focus: Infectious diseases: Vancomycin resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269. [Google Scholar]
- Ferreira, S.L.; Cerda, V.; Cunha, F.A.; Lemos, V.A.; Teixeira, L.S.; dos Santos, W.N.; Coutinho, J.D.; Porto, I.S.d.A.; de Jesus, R.F. Application of human health risk indices in assessing contamination from chemical elements in food samples. TrAC Trends Anal. Chem. 2023, 167, 117281. [Google Scholar] [CrossRef]
- Masson-Matthee, M.D. The Codex Alimentarius Commission and Its Standards; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- CX/CF 12/6/11; FAO/WHO. Food and Agriculture Organization/World Health Organization; Joint FAO/WHO Food Standards Program: Codex Committee on Contaminants in Foods (Editorial Amendments to the General Standard for Contaminants and Toxins in Food and Feed), Sixth Session, Maastricht, The Netherlands, 26–30 March 2012. FAO: Rome, Italy, 2012.
- Khalil, O. Risk Assessment of Certain Heavy Metals and Trace Elements in Milk and Milk Products Consumed in Aswan Province. J. Food Dairy Sci. 2018, 9, 289–296. [Google Scholar] [CrossRef]
- Goldstein, R.E.R.; Micallef, S.A.; Gibbs, S.G.; Davis, J.A.; He, X.; George, A.; Kleinfelter, L.M.; Schreiber, N.A.; Mukherjee, S.; Sapkota, A.; et al. Methicillin-resistant Staphylococcus aureus (MRSA) detected at four US wastewater treatment plants. Environ. Health Perspect. 2012, 120, 1551–1558. [Google Scholar] [CrossRef]
- Alsaadi, L.A.S. Heavy Metals Tolerance and Antibiotics Susceptibilty Profiles of Staphylococcus aureus strains isolated from clinical sources in Baquba city. Diyala J. Pure Sci. 2017, 13, 130–144. [Google Scholar]
- Corey, G.R.; Rubinstein, E.; Stryjewski, M.E.; Bassetti, M.; Barriere, S.L. Potential role for telavancin in bacteremic infections due to gram-positive pathogens: Focus on Staphylococcus aureus. Clin. Infect. Dis. 2015, 60, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, H.; Dehbashi, S.; Arabestani, M.R. Antibiotic resistance alters through iron-regulating Sigma factors during the interaction of Staphylococcus aureus and Pseudomonas aeruginosa. Sci. Rep. 2021, 11, 18509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shen, J.-P.; Zhang, L.-M.; Du, S.; Hu, H.-W.; He, J.-Z. Arsenic and cadmium as predominant factors shaping the distribution patterns of antibiotic resistance genes in polluted paddy soils. J. Hazard. Mater. 2020, 389, 121838. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh, M.; Khoei, A.J.; Parhizkar, S.; Rad, F.T.; Salimi, B. Assessment of some heavy metals and their relationship with oxidative stress and immunological parameters in aquatic animal species. Biol. Trace Elem. Res. 2023, 201, 4547–4557. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.; Almeida, A.; Peixe, L.; Antunes, P.; Novais, C. Unraveling the Role of Metals and Organic Acids in Bacterial Antimicrobial Resistance in the Food Chain. Antibiotics 2023, 12, 1474. [Google Scholar] [CrossRef]
- Fasakin, O. A Metagenomic Guided Survey of Heavy Metals and Antibiotic Resistance Genes in Long-term Contaminated Ecosystems. Ph.D. Thesis, Florida Agricultural and Mechanical University, Tallahassee, FL, USA, 2023. [Google Scholar]
- Grim, K.P.; Francisco, B.S.; Radin, J.N.; Brazel, E.B.; Kelliher, J.L.; Solórzano, P.K.P.; Kim, P.C.; McDevitt, C.A.; Kehl-Fie, T.E. The metallophore staphylopine enables Staphylococcus aureus to compete with the host for zinc and overcome nutritional immunity. mBio 2017, 8, e01281-17. [Google Scholar] [CrossRef] [PubMed]
- Moukafih, B.; El Marrakchi, S.; Bennani, I.; Nchinech, N.; Achour, S.; El Kartouti, A. New antibiotics for multi-drug resistant bacterial strains. Cah. Santé Médecine Thérapeutique 2022, 31, 208–218. [Google Scholar] [CrossRef]
- Grassi, G.; Simonetti, A.; Gambacorta, E.; Perna, A. Effect of species on the distribution and oxidative stability of milk added of lead and cadmium. Ital. J. Anim. Sci. 2023, 22, 1162–1171. [Google Scholar] [CrossRef]
- Su, C.; Gao, Y.; Qu, X.; Zhou, X.; Yang, X.; Huang, S.; Han, L.; Zheng, N.; Wang, J. The occurrence, pathways, and risk assessment of heavy metals in raw milk from industrial areas in China. Toxics 2021, 9, 320. [Google Scholar] [CrossRef]
- Shen, X.; Chi, Y.; Xiong, K. The effect of heavy metal contamination on humans and animals in the vicinity of a zinc smelting facility. PLoS ONE 2019, 14, e0207423. [Google Scholar] [CrossRef]
- Poopak, H.; Raeeszadeh, M.; Salimi, B. Accumulation of heavy metals in meat and their relationship with water and food intake of aquatic animals in Kermanshah, western Iran. Int. J. Environ. Health Res. 2023, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stat, F. Food and Agricultural Organization; United Nation: Rome, Italy, 2017. [Google Scholar]



| Parameters | Value/Type |
|---|---|
| RF generator Power | 1200 W |
| RF frequency | Resonance frequency: 24 MHz |
| Plasma, auxiliary, and nebulizer gas | Argon |
| Plasma gas flow rate | 2/12 (L/min) |
| Auxiliary gas flow rate | 0/8 (L/min) |
| Nebulizer gas flow rate | 0/8 (L/min) |
| Sample uptake time | 260 total (S) |
| Measurement replicate | 3 |
| Type of detector solid state | CCD |
| Type of spray chamber cyclonic | Modified Lichte |
| Metals | Group | Mean (SD) | Mean Difference (CI 95%) | Median (Min-Max) | Statistical Test | p-Value |
|---|---|---|---|---|---|---|
| As | Sensitive | 0.03 (0.02) | (0.02, 0.04) | 0.03 (0.01, 0.06) | t = 8.70 | <0.001 |
| Resistance | 0.11 (0.01) | (0.09, 0.12) | 0.10 (0.10, 0.12) | |||
| Cd | Sensitive | 0.08 (0.03) | (0.06, 0.10) | 0.09 (0.04, 0.10) | z = 0.44 | 0.66 |
| Resistance | 0.07 (0/02) | (0.04, 0.10) | 0.07 (0.05, 0.10) | |||
| Pb | Sensitive | 0.07 (0.03) | (0.05, 0.10) | 0.09 (0.03, 0.10) | z = 0.74 | 0.46 |
| Resistance | 0.06 (0.03) | (0.01, 0.11) | 0.06 (0.03, 0.10) | |||
| Hg | Sensitive | 0.10 (0.01) | (0.09, 0.10) | 0.10 (0.08, 0.10) | z = 1.72 | 0.08 |
| Resistance | 0.09 (0.01) | (0.08, 0.10) | 0.09 (0.08, 0.10) | |||
| Fe | Sensitive | 0.37 (0.23) | (0.21, 0.54) | 0.36 (0.10, 0.75) | t = 2.40 | 0.03 |
| Resistance | 0.09 (0.01) | (0.08, 0.10) | 0.09 (0.08, 0.10) | |||
| Zn | Sensitive | 4.26 (0.61) | (3.82, 4.70) | 4.05 (3.66, 5.66) | z = 2.84 | 0.005 |
| Resistance | 2.89 (0.17) | (2.62, 3.15) | 2.94 (2.65, 3.02) | |||
| Al | Sensitive | 0.48 (0.46) | (0.15, 0.82) | 0.28 (0.10, 1.18) | Z = 2.01 | 0.04 |
| Resistance | 1.02 (0.27) | (0.59, 1.46) | 1.14 (0.62, 1.2) | |||
| Mg | Sensitive | 102.01 (6.59) | (97.29, 106.72) | 102.27 (87.66, 114.93) | z = 0.71 | 0.48 |
| Resistance | 102.80 (11.77) | (84.08, 121.52) | 104.65 (86.90, 115.00) | |||
| Ca | Sensitive | 1332.39 (73.36) | (1279.91, 1384.87) | 1332.39 (1192.20, 1430.73) | t = 0.67 | 0.52 |
| Resistance | 1358.67 (39.21) | (1296.28, 1421.06) | 1363.34 (1306.00, 1400.01) | |||
| K | Sensitive | 1717.57 (101.14) | (1645.22, 1789.92) | 1720.38 (1509.99, 1909.35) | t = 0.82 | 0.43 |
| Resistance | 1763.43 (70.50) | (1651.25, 1875.61) | 1771.72 (1670.00, 1840.29) | |||
| Na | Sensitive | 453.98 (33.24) | (430.2, 477.75) | 453.98 (406.95, 522.48) | t = 2.25 | 0.04 |
| Resistance | 493.26 (12.49) | (473.38, 513.14) | 491.52 (480.00, 510.00) | |||
| S | Sensitive | 186.90 (21.61) | (171.45, 202.36) | 190.86 (158.37, 230.28) | t = 0.37 | 0.72 |
| Resistance | 182.20 (22.07) | (147.07, 217.32) | 181.40 (156.00, 210.00) | |||
| P | Sensitive | 814.32 (55.82) | (774.39, 854.25) | 820.87 (728.16, 891.03) | t = 0.94 | 0.37 |
| Resistance | 786.55 (25.75) | (745.58, 827.52) | 793.35 (750.50, 809.01) |
| Metals | Parameters | Mean (SD) | Max-Min | Statistical Test | p-Value |
|---|---|---|---|---|---|
| As | Food | 0.05 (0.01) | 0.01–0.06 | F = 5.85 | <0.001 |
| Water | 0.82 (0.04) | 0.60–1.0 | |||
| Cd | Food | 0.09 (0.04) | 0.12–0.10 | F = 0.63 | 0.05 |
| Water | 0.08 (0.02) | 0.09–0.01 | |||
| Pb | Food | 0.09 (0.04) | 0.12–0.05 | F = 5.34 | <0.001 |
| Water | 0.04 (0.01) | 0.02-0.09 | |||
| Hg | Food | 0.3 (0.02) | 0.32–0.05 | F = 4.37 | 0.01 |
| Water | 0.06 (0.01) | 0.08–0.02 | |||
| Fe | Food | 0.46 (0.24) | 0.86–0.28 | F = 5.38 | 0.04 |
| Water | 0.07 (0.02) | 0.15-0.06 | |||
| Zn | Food | 3.12 (0.36) | 3.78–3.52 | F = 3.85 | 0.48 |
| Water | 2.15 (0.22) | 3.01–0.68 | |||
| Al | Food | 1.27 (0.51) | 1.63–0.68 | F = 4.25 | 0.032 |
| Water | 1.02 (0.27) | 0.76–0.37 |
| Metals | Group | Mean | Mean Difference (CI 95%) | Limit | Statistical Test | p-Value |
|---|---|---|---|---|---|---|
| As | Sensitive | 0.03 | (0.02, 0.04) | 0.10 | t = 13.56 | 0.012 |
| Resistance | 0.11 | (0.09, 0.12) | 0.10 | t = 14.43 | 0.025 | |
| Cd | Sensitive | 0.08 | (0.06, 0.10) | 0.0026 | w = 0.084 | 0.002 |
| Resistance | 0.07 | (0.04, 0.10) | 0.0026 | t = 6.24 | 0.008 | |
| Pb | Sensitive | 0.07 | (0.05, 0.10) | 0.02 | w = 0.067 | 0.002 |
| Resistance | 0.06 | (0.01, 0.11) | 0.02 | t = 2.72 | 0.07 | |
| Hg | Sensitive | 0.10 | (0.09, 0.10) | 0.01 | w = 0.08 | 0.002 |
| Resistance | 0.09 | (0.08, 0.10) | 0.01 | t = 19.28 | <0.001 | |
| Fe | Sensitive | 0.37 | (0.21, 0.54) | 0.037 | t = 4.61 | 0.001 |
| Resistance | 0.09 | (0.08, 0.10) | 0.037 | t = 12.98 | 0.001 | |
| Zn | Sensitive | 4.26 | (3.82, 4.70) | 0.328 | w = 0.08 | 0.002 |
| Resistance | 2.89 | (2.62, 3.15) | 0.328 | t = 30.50 | <0.001 | |
| Al | Sensitive | 0.48 | (0.15, 0.82) | 0.50 | t = 20.34 | 0.001 |
| Resistance | 1.02 | (0.59, 1.46) | 0.50 | t = 15.29 | 0.002 |
| Parameters | As | Cd | Pb | Hg | Fe | Zn | Al |
|---|---|---|---|---|---|---|---|
| THQ | 0.35942 | 1.58571 | 0.04228 | 0.66247 | 0.000875 | 0.027274 | 0.00133 |
| TR | 161.74 × 10−6 | 967.285 × 10−6 | 1.26 × 10−6 | 298.11 × 10−6 | 919.714 × 10−6 | 2454.68 × 10−6 | 26.64 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeghian, Y.; Raeeszadeh, M.; Karimi Darehabi, H. The Impact of Metal and Heavy Metal Concentrations on Vancomycin Resistance in Staphylococcus aureus within Milk Produced by Cattle Farms and the Health Risk Assessment in Kurdistan Province, Iran. Animals 2024, 14, 148. https://doi.org/10.3390/ani14010148
Sadeghian Y, Raeeszadeh M, Karimi Darehabi H. The Impact of Metal and Heavy Metal Concentrations on Vancomycin Resistance in Staphylococcus aureus within Milk Produced by Cattle Farms and the Health Risk Assessment in Kurdistan Province, Iran. Animals. 2024; 14(1):148. https://doi.org/10.3390/ani14010148
Chicago/Turabian StyleSadeghian, Yeganeh, Mahdieh Raeeszadeh, and Hiva Karimi Darehabi. 2024. "The Impact of Metal and Heavy Metal Concentrations on Vancomycin Resistance in Staphylococcus aureus within Milk Produced by Cattle Farms and the Health Risk Assessment in Kurdistan Province, Iran" Animals 14, no. 1: 148. https://doi.org/10.3390/ani14010148
APA StyleSadeghian, Y., Raeeszadeh, M., & Karimi Darehabi, H. (2024). The Impact of Metal and Heavy Metal Concentrations on Vancomycin Resistance in Staphylococcus aureus within Milk Produced by Cattle Farms and the Health Risk Assessment in Kurdistan Province, Iran. Animals, 14(1), 148. https://doi.org/10.3390/ani14010148