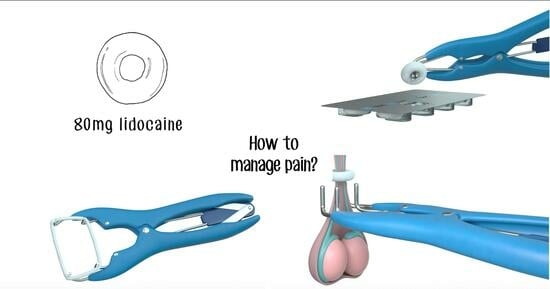
Assessment of the Effective Tissue Concentrations of Injectable Lidocaine and a Lidocaine-Impregnated Latex Band for Castration in Calves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Effective Concentrations (EC50 and EC95) of Injectable Lidocaine in Calf Scrotal Tissue
2.2. In Vivo Delivery of Lidocaine into Scrotal Tissues from LLBs
2.3. Assessment of Tissue Lidocaine Concentrations
2.4. Statistical Analysis
3. Results
3.1. Determination of the Effective Tissue Concentrations of Injectable Lidocaine
3.2. In Vivo Delivery of Lidocaine into Scrotal Tissues from the LLBs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartzkopf-Genswein, K.; Stookey, J.M.; Berg, J.; Campbell, J.; Haley, D.B.; Pajor, E.; McKillop, I. The Code of Practice for the Care and Handling of Beef Cattle: Review of Scientific Research on Priority Issues. 2012. Available online: https://www.nfacc.ca/resources/codes-of-practice/beef-cattle/Beef_Cattle_Review_of_Priority_Welfare_Issues_Nov_2012.pdf (accessed on 19 December 2023).
- Berry, D.P. Invited review: Beef-on-dairy—The generation of crossbred beef x dairy cattle. J. Dairy Sci. 2021, 104, 3789–3819. [Google Scholar] [CrossRef]
- Reed, L.M.; Renaud, D.L.; DeVries, T.J. Male dairy calf welfare: A Canadian perspective on challenges and potential solutions. Can. Vet. J. 2022, 63, 187–193. [Google Scholar]
- Dockweiler, J.C.; Coetzee, J.F.; Edwards-Callaway, L.N.; Bello, N.M.; Glynn, H.D.; Allen, K.A.; Theurer, M.E.; Jones, M.L.; Miller, K.A.; Bergamasco, L. Effect of castration method on neurohormonal and electroencephalographic stress indicators in Holstein calves of different ages. J. Dairy Sci. 2013, 96, 4340–4354. [Google Scholar] [CrossRef] [PubMed]
- The Code of Practice for the Care and Handling of Veal Cattle. Available online: https://www.nfacc.ca/codes-of-practice/veal-cattle (accessed on 11 November 2023).
- Stafford, K.J.; Mellor, D.J.; Todd, S.E.; Bruce, R.A.; Ward, R.N. Effects of local anaesthesia or local anaesthesia plus a non-steriodal anti-inflammatory drug on the acute cortisol response of calves to five different methods of castration. Res. Vet. Sci. 2002, 73, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Ralston, B.; Burwash, L.; Matheson-Bird, H.; Allan, N.D. Efficacy of oral meloxicam suspension for prevention of pain and inflammation following band and surgical castration in calves. BMC Vet. Res. 2016, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Stafford, K.J.; Mellor, D.J. The welfare significance of the castration of cattle: A review. NZ Vet. J. 2005, 53, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Petherick, J.C.; Small, A.H.; Reid, D.J.; Colditz, I.G.; Ferguson, D.M. Welfare outcomes for 3- and 6-month-old beef calves in a tropical environment castrated surgically or by applying rubber rings. Appl. Anim. Behav. Sci. 2015, 171, 47–57. [Google Scholar] [CrossRef]
- Fisher, A.D.; Knight, T.W.; Cosgrove, G.P.; Death, A.F.; Anderson, C.B.; Duganzich, D.M.; Matthews, L.R. Effects of surgical or banding castration on stress responses and behaviour of bulls. Aust. Vet. J. 2001, 79, 279–284. [Google Scholar] [CrossRef] [PubMed]
- González, L.A.; Schwartzkopf-Genswein, K.S.; Caulkett, N.A.; Janzen, E.; McAllister, T.A.; Fierheller, E.; Schaefer, A.L.; Haley, D.B.; Stookey, J.M.; Hendrick, J. Pain mitigation after band castration of beef calves and its effect on performance, behaviour, Escherichia coli, and salivary cortisol. J. Anim. Sci. 2013, 88, 802–810. [Google Scholar] [CrossRef]
- Nogues, E.; von Keyserlingk, M.A.G.; Weary, D.M. Pain in the weeks following surgical and rubber ring castration in dairy calves. J. Dairy Sci. 2021, 104, 12881–12886. [Google Scholar] [CrossRef]
- Dimmitt, S.; Stampfer, H.; Martin, J.H. When less is more—Efficacy with less toxicity at the ED50. Br. J. Clin. Pharmacol. 2017, 83, 1365–1368. [Google Scholar] [CrossRef]
- Nakamura, T.; Popitz-Bergez, F.; Birknes, J.; Strichartz, G.R. The critical role of concentration for lidocaine block of peripheral nerve in vivo: Studies of function and drug uptake in the rate. Anesthesiology 2003, 99, 1189–1197. [Google Scholar] [CrossRef]
- Yartsev, A. Therapeutic Index, ED50, TD50, and LD50. Deranged Physiology, Pharmacodynamics. 2015. Available online: https://derangedphysiology.com/main/cicm-primary-exam/required-reading/pharmacodynamics/Chapter%20413/therapeutic-index-ed50-td50-and-ld50 (accessed on 26 October 2023).
- Yoshida, E.T.K.; Kawaai, H.; Yamazaki, S. Lidocaine Concentration in Oral Tissue by the Addition of Epinephrine. Anesth. Prog. 2016, 63, 17–24. [Google Scholar]
- Irwin, R.J.; Hautus, M.J.; Dawson, N.J.; Welch, D.; Bayly, M.F. Discriminability of electrocutaneous stimuli after topical anesthesia: Detection-theory measurement of sensitivity to painful stimuli. Percep. Psych. 1994, 55, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gordh, T.; Gordh, T.E.; Lindqvist, K. Lidocaine: The origin of a modern local anesthetic. Anesthesiology 2010, 113, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, L.; Peake, B.; Tan, C.; Nikfarjam, M. Pharmacokinetics and pharmacodynamics of lignocaine: A review. World J. Anesthesiol. 2015, 4, 17–29. [Google Scholar] [CrossRef]
- Yang, X.; Wei, X.; Mu, Y.; Li, Q.; Liu, J. A review of the mechanism of the central analgesic effect of lidocaine. Medicine 2020, 99, e19898. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, J.F. A review of pain assessment techniques and pharmacological approaches to pain relief after bovine castration: Practical implications for cattle production within the United States. Appl. Anim. Behav. Sci. 2011, 135, 192–213. [Google Scholar] [CrossRef]
- National Farm Animal Care Council. Code of Practice for the Care and Handling of Sheep. 2013. Available online: https://www.nfacc.ca/pdfs/codes/sheep_code_of_practice.pdf (accessed on 26 October 2023).
- Saville, J.W.; Ross, J.A.; Trefz, T.; Schatz, C.; Matheson-Bird, H.; Ralston, B.; Granot, O.; Schmid, K.; Terry, R.; Allan, N.D.; et al. Development and field validation of lidocaine-loaded castration bands for bovine pain mitigation. Animals 2020, 10, 2363. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Roche, S.M.; Beaugrand, K.; Schaz, C.; Hammad, A.; Ralston, B.J.; Hanson, A.M.; Allan, N.; Olson, M. Assessment of the pharmacokinetics and pharmacodynamics of injectable lidocaine and a lidocaine-impregnated latex band for castration and tail docking in lambs. Animals 2024, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, D.M.; Marti, S.; Pajor, E.A.; Sidhu, P.K.; Gellatly, D.; Moya, D.; Janzen, E.D.; Coetzee, J.F.; Schwartzkopf-Genswein, K.S. Effect of meloxicam and lidocaine administered alone or in combination on indicators of pain and distress during and after knife castration in weaned beef calves. PLoS ONE 2018, 13, e0207289. [Google Scholar] [CrossRef]
- Fierheller, E.E.; Caulkett, N.A.; Haley, D.B.; Florence, D.; Doepel, L. Onset, duration, and efficacy of four methods of local anesthesia. Vet. Anesth. Analg. 2012, 39, 431–435. [Google Scholar] [CrossRef]
- Lomax, S.; Windsor, P.A. Topical anesthesia mitigates the pain of castration in beef calves. J. Anim. Sci. 2013, 91, 2012–5984. [Google Scholar] [CrossRef]
- Warwick, D.N.K.; Ng, F.F.; Khaw, K.S.; Lee, A.; Gin, T. Determination and comparison of graded dose-response curves for epidural bupivacaine and ropivacaine for analgesia in laboring nulliparous women. Anesthesiology 2010, 113, 445–453. [Google Scholar]
- Alonso, M.E.; González-Montaña, J.R.; Lomillos, J.M. Consumers’ concerns and perceptions of farm animal welfare. Animals 2020, 10, 385. [Google Scholar] [CrossRef]
- Stewart, M.; Verkerk, G.A.; Stafford, K.J.; Schaefer, A.L.; Webster, J.R. Noninvasive assessment of autonomic activity of evaluation of pain in calves, using surgical castration as a model. J. Dairy Sci. 2010, 93, 3602–3609. [Google Scholar] [CrossRef] [PubMed]
- Webster, H.B.; Morin, D.; Jarrrell, V.; Shipley, C.; Brown, L.; Green, A.; Wallace, R.; Constable, P.D. Effects of local anesthesia and flunixin meglumine on the acute cortisol response, behavior, and performance of young dairy calves undergoing surgical castration. J. Dairy Sci. 2013, 96, 6285–6300. [Google Scholar] [CrossRef] [PubMed]
- Ede, T.; Nogues, E.; von Keyserlingk, M.A.G.; Weary, D.M. Pain in the hours following surgical and rubber ring castration in dairy calves: Evidence from conditioned place avoidance. JDS Commun. 2022, 3, 421–425. [Google Scholar] [CrossRef] [PubMed]






| Graded Response | Description a |
|---|---|
| 0 | No reaction at Level 10 |
| 1 | Positive reaction at Level 10 |
| 2 | Positive reaction at Level 8 |
| 3 | Positive reaction at Level 6 or below |
| Parameter | Details |
|---|---|
| HPLC | Agilent 1100 and 1200 Series (Agilent Technologies, Santa Clara, CA, USA) |
| Column | ZORBAX Extend-C18; 4.6 × 150 mm; 3.5 µm (Agilent Technologies, Santa Clara, CA, USA) |
| Mobile Phase | 40:60—Acetonitrile:PBS, pH 7.4 |
| Analysis Time | 15 min |
| Flow Rate | 1.0 mL/min |
| Injection Volume | 10 µL |
| Column Temperature | 28 °C |
| Detector | Agilent G1315B Diode Array Detector (DAD) (Agilent Technologies, Santa Clara, CA, USA) |
| Wavelength | 210 nm |
| Bandwidth | 4 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, J.A.; Roche, S.M.; Beaugrand, K.; Schatz, C.; Hammad, A.; Ralston, B.J.; Hanson, A.M.; Allan, N.; Olson, M. Assessment of the Effective Tissue Concentrations of Injectable Lidocaine and a Lidocaine-Impregnated Latex Band for Castration in Calves. Animals 2024, 14, 977. https://doi.org/10.3390/ani14060977
Ross JA, Roche SM, Beaugrand K, Schatz C, Hammad A, Ralston BJ, Hanson AM, Allan N, Olson M. Assessment of the Effective Tissue Concentrations of Injectable Lidocaine and a Lidocaine-Impregnated Latex Band for Castration in Calves. Animals. 2024; 14(6):977. https://doi.org/10.3390/ani14060977
Chicago/Turabian StyleRoss, Joseph A., Steven M. Roche, Kendall Beaugrand, Crystal Schatz, Ann Hammad, Brenda J. Ralston, Andrea M. Hanson, Nicholas Allan, and Merle Olson. 2024. "Assessment of the Effective Tissue Concentrations of Injectable Lidocaine and a Lidocaine-Impregnated Latex Band for Castration in Calves" Animals 14, no. 6: 977. https://doi.org/10.3390/ani14060977
APA StyleRoss, J. A., Roche, S. M., Beaugrand, K., Schatz, C., Hammad, A., Ralston, B. J., Hanson, A. M., Allan, N., & Olson, M. (2024). Assessment of the Effective Tissue Concentrations of Injectable Lidocaine and a Lidocaine-Impregnated Latex Band for Castration in Calves. Animals, 14(6), 977. https://doi.org/10.3390/ani14060977