Use of Anecdotal Occurrence Data in Species Distribution Models: An Example Based on the White-Nosed Coati (Nasua narica) in the American Southwest
Abstract
:Simple Summary
Abstract
1. Introduction
| Class | Characteristics |
|---|---|
| Reliability | |
| A | Verified: An expert’s evaluation of preserved physical evidence, including photographs. |
| B | Highly Probable: An expert’s accurate observation, but no physical evidence is preserved. |
| C | Probable: A first-hand report of an observation that is likely to be accurate. Convincing details are provided. |
| D | Possible: A potentially inaccurate observation made by an expert due to poor conditions. |
| E | Questionable: First-hand report of a potentially inaccurate observation because of the observer’s lack of knowledge, suboptimal observation conditions, or the lack of supporting details, this class is not as convincing as class C. |
| F | Highly Questionable: Records that have a high potential of inaccuracy. Includes second-hand and unpublished reports. |
| G | Erroneous: Physical evidence verifies the reported species was misidentified. |
| Precision | |
| H | Actual location likely <30 m of coordinate |
| I | Actual location likely 30–500 m of coordinate |
| J | Actual location likely 500–1,000 m of coordinate |
| K | Actual location likely 1,000–2,000 m of coordinate |
| L | Actual location likely 2,000–3,000 m of coordinate |
| M | Actual location likely >3,000 m of coordinate |
2. Methods
2.1. Occurrence Records
| Reliability | |||||||
|---|---|---|---|---|---|---|---|
| Precision | A | B | C | D | E | F | total |
| H | 18 | 58 | 10 | 1 | 3 | 2 | 92 |
| I | 12 | 33 | 13 | 0 | 7 | 7 | 72 |
| J | 9 | 17 | 10 | 1 | 3 | 5 | 45 |
| K | 6 | 14 | 4 | 0 | 2 | 4 | 30 |
| L | 4 | 11 | 1 | 0 | 0 | 1 | 17 |
| M | 6 | 31 | 7 | 3 | 3 | 11 | 61 |
| total | 55 | 164 | 45 | 5 | 18 | 30 | 317 |

| Models | Occurrence records 1 | AUC | Variable contributions (%) 2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Name | Reliability | Precision | N | Mean Training | Mean Test | SD | Bio 3 | Bio 4 | Bio 6 | Bio 8 | Bio 9 | Bio 10 | Bio 13 | Bio 14 | Bio 15 | Bio 16 | Bio 17 | Bio19 |
| 1 | Very Conservative | A | H | 18 | 0.945 | 0.933 | 0.025 | 39.0 ** | 5.8 * | 19.5 | 1.8 | 22.7 | 1.2 | 5.1 | 4.9 | ||||
| 2 | Conservative | A | H-I | 30 | 0.967 | 0.935 | 0.029 | 22.9 ** | 14.3 * | 3.9 | 21.5 | 2.4 | 14.6 | 7.2 | 1.0 | 12.3 | |||
| 3 | Best A Priori | A-B | H-I | 103 | 0.967 | 0.956 | 0.013 | 32.3 * | 13.7 ** | 2.3 | 19.3 | 0.9 | 14.8 | 6.4 | 0.4 | 10.0 | |||
| 4 | Moderate | A-C | H-J | 153 | 0.971 | 0.954 | 0.013 | 37.7 * | 8.8 ** | 4.1 | 14.2 | 1.9 | 11.7 | 7.9 | 0.5 | 13.2 | |||
| 5 | Liberal | A-F | H-M | 279 | 0.958 | 0.934 | 0.014 | 38.5 * | 12.1 ** | 4.5 | 13.2 | 2.6 | 12.2 | 5.5 | 1.2 | 10.2 | |||
| 6 | Poor Reliability | C-F | H-I | 42 | 0.950 | 0.909 | 0.038 | 63.2 * | 2.4 ** | 0.5 | 15.0 | 3.3 | 7.3 | 4.7 | 0.3 | 3.2 | |||
| 7 | Poor Precision | A-B | J-M | 89 | 0.955 | 0.944 | 0.022 | 19.2 * | 12 ** | 15.4 | 5.9 | 14.3 | 7.7 | 3.0 | 22.5 | ||||
| Models | Occurrence records 1 | AUC | Variable contributions (%) 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Name | Reliability | Precision | N | Mean Training | Mean Test | SD | Land-cover | Distance To Springs | Distance To Streams | Distance To Lakes | Slope | Elevation | Road Density | ||
| 8 | Very Conservative | A | H | 18 | 0.972 | 0.878 | 0.067 | 50.7 * | 27.1 ** | 2.1 | 6.2 | 1.0 | 0.3 | 12.7 | ||
| 9 | Conservative | A | H-I | 30 | 0.969 | 0.920 | 0.041 | 2.9 * | 26.2 * | 16.3 | 12.4 | 9.6 | 5.7 | 26.9 ** | ||
| 10 | Best A Priori | A-B | H-I | 115 | 0.974 | 0.941 | 0.082 | 28.6 * | 26.2 ** | 9.7 | 6.6 | 12.1 | 9.5 | 7.3 | ||
| 11 | Moderate | A-C | H-J | 168 | 0.965 | 0.934 | 0.018 | 26.7 * | 28.2 ** | 12.1 | 6.7 | 9.4 | 8.4 | 8.3 | ||
| 12 | Liberal | A-F | H-M | 299 | 0.954 | 0.926 | 0.015 | 30.6 * | 24.1 ** | 11.6 | 3.4 | 10.1 | 8.7 | 11.5 | ||
| 13 | Poor Reliability | C-F | H-I | 42 | 0.953 | 0.876 | 0.046 | 37.7 * | 22.3 ** | 7.7 | 5.6 | 7.4 | 5.6 | 13.8 | ||
| 14 | Poor Precision | A-B | J-M | 91 | 0.965 | 0.937 | 0.022 | 51.4 * | 13.4 ** | 10.9 | 1.5 | 7.0 | 3.3 | 12.5 | ||
2.2. Model Development
2.3. Model Evaluations and Comparisons
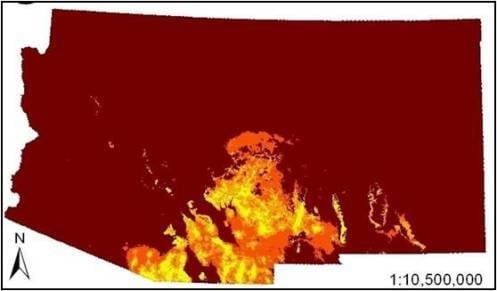
3. Results
| Land-cover type | Proportion of area of suitable habitat (%) 1 | Mean habitat suitability (%) |
|---|---|---|
| Madrean Encinal | 6.3 | 47.1 |
| Madrean Pinyon-Juniper Woodland | 12.6 | 40.7 |
| Mogollon Chaparral | 2.5 | 38.8 |
| Chihuahuan Mixed Salt Desert Scrub | 2.5 | 34.7 |
| Madrean Lower Montane Pine-Oak Forest and Woodland | 1.4 | 34.6 |
| Apacherian-Chihuahuan Mesquite Upland Scrub | 10.6 | 22.6 |
| Apacherian-Chihuahuan Semi-Desert Grassland and Steppe | 28.2 | 18.9 |
| Chihuahuan Creosote, Mixed Desert and Thorn Scrub | 10.4 | 16.7 |
| Southern Rocky Mountain Ponderosa Pine Woodland | 8.5 | 14.6 |
| Colorado Plateau Pinyon-Juniper Woodland | 5.1 | 13.9 |
| Chihuahuan Stabilized Coppice Dune and Sand Flat Scrub | 1.2 | 8.3 |
| Sonoran Paloverde-Mixed Cacti Desert Scrub | 2.8 | 5.9 |

4. Discussion
4.1. Influence of Reliability of Occurrence Records
4.2. Determinants of Coati Distribution


4.4. Structure of Coati Distribution at its Range Margin
| Land-cover | Arizona (N = 13) | New Mexico (N = 90) | Total (N = 103) |
|---|---|---|---|
| Madrean Pinyon-Juniper Woodland | 15.4 | 28.9 | 27.2 |
| Apacherian-Chihuahuan Piedmont Semi-Desert Grassland and Steppe | 0.0 | 20.0 | 17.5 |
| Madrean Encinal | 30.8 | 11.1 | 13.6 |
| Mogollon Chaparral | 15.4 | 6.7 | 7.8 |
| other | 0.0 | 8.9 | 7.8 |
| Apacherian-Chihuahuan Mesquite Upland Scrub | 7.7 | 6.7 | 6.8 |
| Rocky Mountain Ponderosa Pine Woodland | 7.7 | 6.7 | 6.8 |
| Chihuahuan Mixed Salt Desert Scrub | 7.7 | 4.4 | 4.9 |
| Madrean Pine-Oak Forest and Woodland | 7.7 | 2.2 | 2.9 |
| Chihuahuan Creosotebush, Mixed Desert and Thorn Scrub | 0.0 | 2.2 | 1.9 |
| Chihuahuan Stabilized Coppice Dune and Sand Flat Scrub | 0.0 | 1.1 | 1.0 |
| Colorado Plateau Pinyon-Juniper Woodland | 0.0 | 1.1 | 1.0 |
| Sonoran Paloverde-Mixed Cacti Desert Scrub | 7.7 | 0.0 | 1.0 |
5. Conclusions

Acknowledgements
Conflict of Interest
References
- McKelvey, K.S.; Aubry, K.B.; Schwartz, M.K. Using anecdotal occurrence data for rare or elusive species: The illusion of reality and a call for evidentiary standards. Bioscience 2008, 58, 549–555. [Google Scholar] [CrossRef]
- Frey, J.K. Inferring species distributions in the absence of occurrence records: An example considering wolverine (Gulo gulo) and canada lynx (Lynx canadensis) in New Mexico. Biol. Conserv. 2006, 130, 16–24. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H. Do they? How do they? Why do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Aubry, K.B.; Jagger, L.A. Importance of obtaining verifiable occurrence data on forest carnivores and an interactive website for archiving results from standardized surveys. In Martes in Carnivore Communities; Santos-Reis, M., Birks, D.S., O’Doherty, E.C., Proulx, G., Eds.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 2006; pp. 159–176. [Google Scholar]
- Braunisch, V.; Suchant, R. Predicting species distributions based on incomplete survey data: The trade-off between precision and scale. Ecography 2010, 33, 826–840. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar]
- Hernandez, P.A.; Franke, I.; Herzog, S.K.; Pacheco, V.; Paniagua, L.; Quintana, H.L.; Soto, A.; Swenson, J.J.; Tovar, C.; Valqui, T.H.; et al. Predicting species distributions in poorly-studied landscapes. Biodiv. Conserv. 2008, 17, 1353–1366. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A. NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Peterson, A.T.; Loiselle, B.A. NCEAS Species Distribution Modeling Group. The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 2008, 45, 239–247. [Google Scholar]
- Lozier, J.D.; Aniello, P.; Hickerson, M.J. Predicting the distribution of Sasquatch in western North America: Anything goes with ecological niche modelling. J. Biogeogr. 2009, 36, 1623–1627. [Google Scholar] [CrossRef]
- Aubry, K.B.; Houston, D.B. Distribution and status of the fisher (martes pennanti) in washington. Northwestern Naturalist 1992, 73, 69–79. [Google Scholar] [CrossRef]
- Aubry, K.B.; Lewis, J.C. Extirpation and reintroduction of fishers (Martes pennanti) in Oregon: Implications for their conservation in the pacific states. Biol. Conserv. 2003, 114, 79–90. [Google Scholar] [CrossRef]
- Gompper, M.E. Nasua narica. Mammal. Species 1995, 487, 1–10. [Google Scholar] [CrossRef]
- Cahalane, V.H. Mammals of the Chiricahua Mountains, Cochise County, Arizona. J. Mammal. 1939, 20, 418–440. [Google Scholar] [CrossRef]
- Taylor, W.P. Coati added to the list of United States mammals. J. Mammal. 1934, 15, 317–318. [Google Scholar]
- Tabor, F.W. Range of the coati in the United States. J. Mammal. 1940, 21, 11–14. [Google Scholar] [CrossRef]
- Wallmo, O.C.; Gallizioli, S. Status of the coati in Arizona. J. Mammal. 1954, 35, 48–54. [Google Scholar] [CrossRef]
- Wetherhill, M.A. Occurrence of coati in northern Arizona. J. Mammal. 1957, 38, 123. [Google Scholar] [CrossRef]
- Kaufmann, J.H.; Lanning, D.V.; Poole, S.E. Current status and distribution of coati in United States. J. Mammal. 1976, 57, 621–637. [Google Scholar] [CrossRef]
- Hoffmeister, D.F. Mammals of Arizona; University of Arizona Press: Tucson, AZ, USA, 1986; p. 602. [Google Scholar]
- Brown, D.E.; Davis, R. One hundred years of vicissitude: Terrestrial bird and mammal distribution changes in the american southwest, 1890–1990. In Biodiversity and Management of the Madrean Archipelago: The Sky Islands of Southwestern United States and Northwestern Mexico. 1994 September 19–23;Tucson, AZ; General Technical Report RM-GTR-264; Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1995; p. 669. [Google Scholar]
- Taber, F.W. Range of the coati in the United States. J. Mammal. 1940, 21, 11–14. [Google Scholar] [CrossRef]
- Davis, R.; Callahan, J.R. Post-pleistocene dispersal in the Mexican vole (Microtus mexicanus): An example of an apparent trend in the distribution of southwestern mammals. Great Basin Naturalist 1992, 52, 262–268. [Google Scholar]
- Cook, J.A. The Mammals of the Animas Mountains and Adjacent Areas, Hidalgo County, New Mexico; Occasional Papers; Museum of Southwestern Biology, University of New Mexico: Albuquerque, NM, USA, 1986; Volume 4, pp. 1–45. [Google Scholar]
- Feeley, K.J.; Silman, M.R. The data void in modeling current and future distributions of tropical species. Global Change Biol. 2011, 17, 626–630. [Google Scholar] [CrossRef]
- Tingley, R.; Herman, T.B. Land-cover data improve bioclimatic models for anurans and turtles at a regional scale. J. Biogeogr. 2009, 36, 1656–1672. [Google Scholar] [CrossRef]
- Calkins, M.T.; Beever, E.A.; Boykin, K.G.; Frey, J.K.; Andersen, M.C. Not-so-splendid isolation: Modeling climate-mediated range collapse of a montane mammal ochotona princeps across numerous ecoregions. Ecography 2012, 35, 780–791. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- USGS National Gap Analysis Program, Provisional Digital Land Cover Map for the Southwestern United States, Version 1.0; RS/GIS Laboratory, College of Natural Resources, Utah State University: Logan, UT, USA, 2004.
- USGS, National Elevation Dataset, U.S. Geological Survey: Washington, DC, USA, 1999.
- Files, T.L. Census 2000 Tiger/Line Files, (Machine-Readable Data Files); U.S. Census Bureau: Washington, DC, USA, 2000.
- USEP Agency, National Hydrography Dataset Plus, Version 1.0.; 2006 ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- The R Development Core Team. R: A Language and Environment for Statistical Computing, Version 2.12.0; 2010. Available online: http://www.r-project.org/ (accessed on 5 April 2013).
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under the receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar]
- Raxworthy, C.J.; Martinez-Meyer, E.; Horning, N.; Nussbaum, R.A.; Schneider, G.E.; Ortega-Huerta, M.A.; Peterson, A.T. Predicting distributions of known and unknown reptile species in Madagascar. Nature 2003, 426, 837–841. [Google Scholar]
- Jackson, C.R.; Robertson, M.P. Predicting the potential distribution of an endangered cryptic subterranean mammal from few occurrence records. J. Nat. Conserv. 2011, 19, 87–94. [Google Scholar] [CrossRef]
- Muldavin, E.H.; DeVelice, R.L.; Ronco, F., Jr. A Classification of Forest Habitat Types Southern Arizona and Portions of the Colorado Plateau; USDA, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1996; p. 130. [Google Scholar]
- Brown, D.E. Biotic Communities Southwestern United States and Northwestern Mexico; University of Utah Press: Salt Lake City, UT, USA, 1994; p. 342. [Google Scholar]
- Hass, C.C. Home-range dynamics of white-nosed coatis in southeastern Arizona. J. Mammal. 2002, 83, 934–946. [Google Scholar] [CrossRef]
- Lanning, D.V. Density and movements of the coati in Arizona. J. Mammal. 1976, 57, 609–611. [Google Scholar] [CrossRef]
- McColgin, M.E. Sociality and Genetics of a Southeastern Arizona Coati (Nasua narica) Population; Purdue University: West Lafayette, IN, USA, 2006. [Google Scholar]
- Valenzuela, D.; Macdonald, D.W. Home-range use by white-nosed coatis (Nasua narica): Limited water and a test of the resource dispersion hypothesis. J. Zool. 2002, 258, 247–256. [Google Scholar] [CrossRef]
- Hengeveld, R. Dynamic Biogeography; Cambridge University Press: Cambridge, UK, 1990; p. 192. [Google Scholar]
- Pulliam, H.R. On the relationship between niche and distribution. Ecol. Lett. 2000, 3, 349–361. [Google Scholar]
- Gaston, K.J. The Structure and Dynamics of Geographic Ranges; Oxford University Press: New York, NY, USA, 2003; p. 280. [Google Scholar]
- Ratnayeke, S.; Bixler, A.; Gitleman, J.L. Home range movements of solitary, reprodutive female coatis, Nasua narica, in south-eastern Arizona. J. Zool. 1994, 233, 322–326. [Google Scholar] [CrossRef]
- Gompper, M.E.; Gittleman, J.L.; Wayne, R.K. Dispersal, philopatry, and genetic relatedness in a social carnivore: Comparing males and females. Mol. Ecol. 1998, 7, 157–163. [Google Scholar] [CrossRef]
- Hass, C.C.; Valenzuela, D. Anti-predator benefits of group living in white-nosed coatis (Nasua narica). Behav. Ecol. Sociobiol. 2002, 51, 570–578. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Frey, J.K.; Lewis, J.C.; Guy, R.K.; Stuart, J.N. Use of Anecdotal Occurrence Data in Species Distribution Models: An Example Based on the White-Nosed Coati (Nasua narica) in the American Southwest. Animals 2013, 3, 327-348. https://doi.org/10.3390/ani3020327
Frey JK, Lewis JC, Guy RK, Stuart JN. Use of Anecdotal Occurrence Data in Species Distribution Models: An Example Based on the White-Nosed Coati (Nasua narica) in the American Southwest. Animals. 2013; 3(2):327-348. https://doi.org/10.3390/ani3020327
Chicago/Turabian StyleFrey, Jennifer K., Jeremy C. Lewis, Rachel K. Guy, and James N. Stuart. 2013. "Use of Anecdotal Occurrence Data in Species Distribution Models: An Example Based on the White-Nosed Coati (Nasua narica) in the American Southwest" Animals 3, no. 2: 327-348. https://doi.org/10.3390/ani3020327
APA StyleFrey, J. K., Lewis, J. C., Guy, R. K., & Stuart, J. N. (2013). Use of Anecdotal Occurrence Data in Species Distribution Models: An Example Based on the White-Nosed Coati (Nasua narica) in the American Southwest. Animals, 3(2), 327-348. https://doi.org/10.3390/ani3020327