Characterization of Extracellular Vesicles from Cilia and Epithelial Cells of Ductuli Efferentes in a Turtle (Pelodiscus sinensis)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Animals
2.2. Light Microscopy
2.3. CD63 Immunohistochemistry
2.4. Transmission Electron Microscopy (TEM)
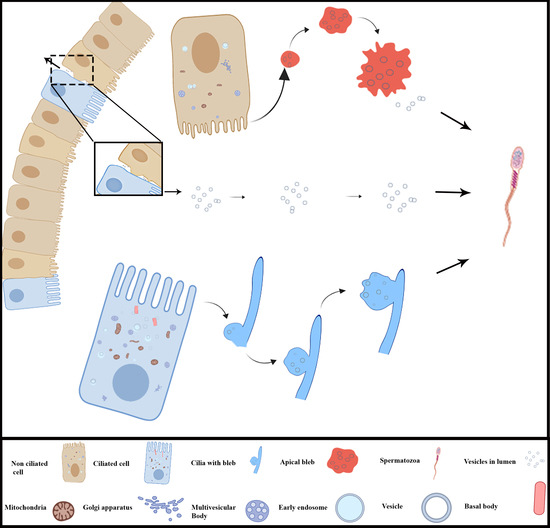
3. Results
3.1. CD63 Immunohistochemistry of Ductuli Efferentes in Chinese Soft-Shelled Turtle
3.2. Ultrastructure of Ductuli Efferentes and Distribution of Early Endosomes and Multivesicular Bodies
3.3. Extracellular Vesicles and Their Interaction with Spermatozoa in the Lumen of Ductuli Efferentes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bian, X.; Gandahi, J.A.; Liu, Y.; Yang, P.; Liu, Y.; Zhang, L.; Zhang, Q.; Chen, Q. The ultrastructural characteristics of the spermatozoa stored in the cauda epididymidis in Chinese soft-shelled turtle Pelodiscus sinensis during the breeding season. Micron 2013, 44, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, X.K.; Qi, Y.Y.; Liu, Y.; Chen, Q.S. Seasonal effects on apoptosis and proliferation of germ cells in the testes of the Chinese soft-shelled turtle, Pelodiscus sinensis. Theriogenology 2008, 69, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Badran, H.H.; Hermo, L.S. Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J. Androl. 2002, 23, 358–373. [Google Scholar] [PubMed]
- Waqas, M.Y.; Liu, T.; Yang, P.; Ahmed, N.; Zhang, Q.; Hu, L.; Hong, C.; Chen, Q. Morphological and ultrastructural study of the efferent ductules in the Chinese soft-shelled turtle Pelodiscus sinensis. J. Exp. Zool A Ecol. Genet. Physiol. 2016, 325, 122–131. [Google Scholar] [CrossRef]
- Clulow, J.; Jones, R.C.; Hansen, L.A.; Man, S.Y. Fluid and electrolyte reabsorption in the ductuli efferentes testis. J. Reprod Fertil Suppl. 1998, 53, 1–14. [Google Scholar]
- Yuan, S.; Liu, Y.; Peng, H.; Tang, C.; Hennig, G.W.; Wang, Z.; Wang, L.; Yu, T.; Klukovich, R.; Zhang, Y.; et al. Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence. Proc. Natl. Acad. Sci. USA 2019, 116, 3584–3593. [Google Scholar] [CrossRef] [Green Version]
- Hess, R.A. Efferent Ductules: Structure and Function. In Encyclopedia of Reproduction (Second Edition); Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; pp. 270–278. [Google Scholar]
- Hess, R.A.; Bunick, D.; Lee, K.H.; Bahr, J.; Taylor, J.A.; Korach, K.S.; Lubahn, D.B. A role for oestrogens in the male reproductive system. Nature 1997, 390, 509–512. [Google Scholar] [CrossRef] [Green Version]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A Common Pathway for a Specialized Function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Saleem, S.N.; Abdel-Mageed, A.B. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol. Life Sci. 2015, 72, 1–10. [Google Scholar] [CrossRef]
- Duijvesz, D.; Versluis, C.Y.L.; van der Fels, C.A.M.; Vredenbregt-van den Berg, M.S.; Leivo, J.; Peltola, M.T.; Bangma, C.H.; Pettersson, K.S.I.; Jenster, G. Immuno-based detection of extracellular vesicles in urine as diagnostic marker for prostate cancer. Int. J. Cancer 2015, 137, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef] [PubMed]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Kamiguchi, Y.; Mikamo, K.; Suzuki, F.; Yanagimachi, H. Maturation of Spermatozoa in the Epididymis of the Chinese-Hamster. Am. J. Anat. 1985, 172, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015, 22, 34–45. [Google Scholar] [CrossRef]
- Fevrier, B.; Raposo, G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004, 16, 415–421. [Google Scholar] [CrossRef]
- Schorey, J.S.; Bhatnagar, S. Exosome function: From tumor immunology to pathogen biology. Traffic 2008, 9, 871–881. [Google Scholar] [CrossRef]
- Menad, R.; Smaï, S.; Moudilou, E.; Khammar, F.; Exbrayat, J.-M.; Gernigon-Spychalowicz, T. Immunolocalization of estrogen and androgen receptors in the caput epididymidis of the fat sand rat (Psammomys obesus): Effects of seasonal variations, castration and efferent duct ligation. Acta Histochem. 2014, 116, 559–569. [Google Scholar] [CrossRef]
- Ritzén, E.M.; French, F.S. Demonstraion of an adrogen binding protein (ABP) in rabbit testis: Secretion in efferent duct fluid and passage into epididymis. J. Steroid Biochem. 1974, 5, 151–154. [Google Scholar] [CrossRef]
- Suzuki, F. Microvasculature of the mouse testis and excurrent duct system. Am. J. Anat. 1982, 163, 309–325. [Google Scholar] [CrossRef]
- Wakui, S.; Furusato, M.; Takahashi, H.; Motoya, M.; Ushigome, S. Lectin histochemical evaluation of glycoconjugates in dog efferent ductules. J. Anat. 1996, 188, 541–546. [Google Scholar]
- Hermo, L.; Clermont, Y.; Morales, C. Fluid-phase and adsorptive endocytosis in ciliated epithelial cells of the rat ductuli efferentes. Anat. Rec. 1985, 211, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Rheubert, J.L.; Sever, D.M.; Geheber, A.D.; Siegel, D.S. Proximal Testicular Ducts of the Mediterranean Gecko (Hemidactylus turcicus). Anat. Rec. 2010, 293, 2176–2192. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.M.; Calderon, M.L.; de Perez, G.R.; Ramirez Pinilla, M.P. Morphology of the male reproductive duct system of Caiman crocodilus (Crocodylia, Alligatoridae). Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2004, 186, 235–245. [Google Scholar] [CrossRef]
- Holmes, H.J.; Gist, D.H. Excurrent duct system of the male turtle Chrysemys picta. J. Morphol. 2004, 261, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Akbarsha, M.A.; Kadalmani, B.; Tamilarasan, V. Efferent ductules of the fan-throated lizard Sitana ponticeriana Cuvier: Light and transmission electron microscopy study. Acta Zool. 2007, 88, 265–274. [Google Scholar] [CrossRef]
- Johnstone, R.M. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006, 36, 315–321. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef]
- Chen, Q.; Takada, R.; Noda, C.; Kobayashi, S.; Takada, S. Different populations of Wnt-containing vesicles are individually released from polarized epithelial cells. Sci. Rep. 2016, 6, 35562. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell Proteom. 2013, 12, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kassianos, A.J.; Kildey, K.; Potriquet, J.; Wilkinson, R.; Healy, H. Analysis of Apical and Basolateral Secreted Exosomes from Human Proximal Tubule Epithelial Cells (Ptec) under Normal and Diseased Conditions. Nephrology 2017, 22, 36. [Google Scholar]
- Aumüller, G.; Renneberg, H.; Schiemann, P.J.; Wilhelm, B.; Seitz, J.; Konrad, L.; Wennemuth, G. The Role of Apocrine Released Proteins in the Post-Testicular Regulation of Human Sperm Function. In The Fate of the Male Germ Cell; Ivell, R., Holstein, A.-F., Eds.; Springer: Boston, MA, USA, 1997; pp. 193–219. [Google Scholar]
- Hermo, L.; Jacks, D. Nature’s ingenuity: Bypassing the classical secretory route via apocrine secretion. Mol. Reprod. Dev. 2002, 63, 394–410. [Google Scholar] [CrossRef]
- Sullivan, R.; D’Amours, O.; Caballero, J.; Belleannée, C. The sperm journey in the excurrent duct: Functions of microvesicles on sperm maturation and gene expression along the epididymis. Anim. Reprod. 2015, 12, 88–92. [Google Scholar]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21. [Google Scholar] [CrossRef]
- Chen, H.; Yang, P.; Chu, X.; Huang, Y.; Liu, T.; Zhang, Q.; Li, Q.; Hu, L.; Waqas, Y.; Ahmed, N.; et al. Cellular evidence for nano-scale exosome secretion and interactions with spermatozoa in the epididymis of the Chinese soft-shelled turtle, Pelodiscus sinensis. Oncotarget 2016, 7, 19242–19250. [Google Scholar] [CrossRef]
- Hansen, L.A.; Clulow, J.; Jones, R.C. The role of Na+-H+ exchange in fluid and solute transport in the rat efferent ducts. Exp. Physiol. 1999, 84, 521–527. [Google Scholar]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Rivas, A.; Fisher, J.S.; McKinnell, C.; Atanassova, N.; Sharpe, R.M. Induction of Reproductive Tract Developmental Abnormalities in the Male Rat by Lowering Androgen Production or Action in Combination with a Low Dose of Diethylstilbestrol: Evidence for Importance of the Androgen-Estrogen Balance. Endocrinology 2002, 143, 4797–4808. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarique, I.; Liu, Y.; Bai, X.; Haseeb, A.; Yang, P.; Huang, Y.; Qu, W.; Wu, R.; Vistro, W.A.; Chen, Q. Characterization of Extracellular Vesicles from Cilia and Epithelial Cells of Ductuli Efferentes in a Turtle (Pelodiscus sinensis). Animals 2019, 9, 888. https://doi.org/10.3390/ani9110888
Tarique I, Liu Y, Bai X, Haseeb A, Yang P, Huang Y, Qu W, Wu R, Vistro WA, Chen Q. Characterization of Extracellular Vesicles from Cilia and Epithelial Cells of Ductuli Efferentes in a Turtle (Pelodiscus sinensis). Animals. 2019; 9(11):888. https://doi.org/10.3390/ani9110888
Chicago/Turabian StyleTarique, Imran, Yifei Liu, Xuebing Bai, Abdul Haseeb, Ping Yang, Yufei Huang, Wenjia Qu, Ruizhi Wu, Waseem Ali Vistro, and Quisheng Chen. 2019. "Characterization of Extracellular Vesicles from Cilia and Epithelial Cells of Ductuli Efferentes in a Turtle (Pelodiscus sinensis)" Animals 9, no. 11: 888. https://doi.org/10.3390/ani9110888
APA StyleTarique, I., Liu, Y., Bai, X., Haseeb, A., Yang, P., Huang, Y., Qu, W., Wu, R., Vistro, W. A., & Chen, Q. (2019). Characterization of Extracellular Vesicles from Cilia and Epithelial Cells of Ductuli Efferentes in a Turtle (Pelodiscus sinensis). Animals, 9(11), 888. https://doi.org/10.3390/ani9110888