Immunohistochemical Detection of Encephalitozoon cuniculi in Ocular Structures of Immunocompetent Rabbits
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of E. cuniculi Spores
2.2. Animals and Experimental Design
2.3. Immunohistochemistry
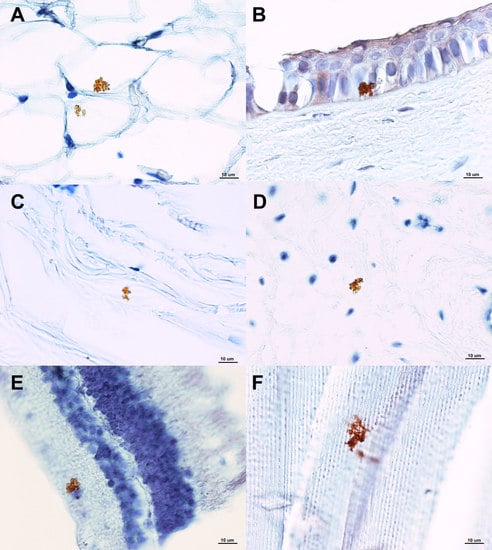
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wright, J.H.; Craighead, E.M. Infectious motor paralysis in young rabbits. J. Exp. Med. 1922, 36, 135–140. [Google Scholar] [CrossRef]
- Levaditi, C.; Nicolau, S.; Schoen, R. L’étiologie de l´encephalite. C. R. Hebd. Seances Acad. Sci. 1923, 177, 985–988. [Google Scholar]
- Mathis, A.; Weber, R.; Deplazes, P. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 2005, 18, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Mathis, A.; Baumgartner, R.; Tanner, I.; Weber, R. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin. Infect. Dis. 1996, 22, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S.; Didier, P.J.; Snowden, K.F.; Shadduck, J.A. Microsporidiosis in mammals. Microbes Infect. 2000, 2, 709–720. [Google Scholar] [CrossRef]
- Shadduck, J.A.; Pakes, S.P. Encephalitozoonosis (nosematosis) and toxoplasmosis. Am. J. Pathol. 1971, 64, 657–673. [Google Scholar] [PubMed]
- Dipineto, L.; Rinaldi, L.; Santaniello, A.; Sensale, M.; Cuomo, A.; Calabria, M.; Menna, L.F.; Fioretti, A. Serological survey for antibodies to Encephalitozoon cuniculi in pet rabbits in Italy. Zoonoses Public Health 2008, 55, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Künzel, F.; Gruber, A.; Tichy, A.; Edelhofer, R.; Nell, B.; Hassan, J.; Leschnik, M.; Thalhammer, J.G.; Joachim, A. Clinical symptoms and diagnosis of encephalitozoonosis in pet rabbits. Vet. Parasitol. 2008, 151, 115–124. [Google Scholar] [CrossRef]
- Jeklova, E.; Jekl, V.; Kovarcik, K.; Hauptman, K.; Koudela, B.; Neumayerova, H.; Knotek, Z.; Faldyna, M. Usefulness of detection of specific IgM and IgG antibodies for diagnosis of clinical encephalitozoonosis in pet rabbits. Vet. Parasitol. 2010, 170, 143–148. [Google Scholar] [CrossRef]
- Hunt, R.D.; King, N.W.; Foster, H.L. Encephalitozoonosis: Evidence for vertical transmission. J. Infect. Dis. 1972, 126, 212–214. [Google Scholar] [CrossRef]
- Baneux, P.J.; Pognan, F. In utero transmission of Encephalitozoon cuniculi strain type I in rabbits. Lab. Anim. 2003, 37, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, O.; Karagoz, A.; Kocak, N. First molecular evidence of ocular transmission of Encephalitozoonosis during the intrauterine period in rabbits. Parasitol. Int. 2019, 71, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jeklova, E.; Leva, L.; Kovarcik, K.; Matiasovic, J.; Kummer, V.; Maskova, J.; Skoric, M.; Faldyna, M. Experimental oral and ocular Encephalitozoon cuniculi infection in rabbits. Parasitology 2010, 137, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S.; Stovall, M.E.; Green, L.C.; Brindley, P.J.; Sestak, K.; Didier, P.J. Epidemiology of microsporidiosis: Sources and modes of transmission. Vet. Parasitol. 2004, 126, 145–166. [Google Scholar] [CrossRef]
- Cox, J.C.; Hamilton, R.C.; Attwood, H.D. An investigation of the route and progression of Encephalitozoon cuniculi infection in adult rabbits. J. Protozool. 1979, 26, 260–265. [Google Scholar] [CrossRef]
- Wolfer, J.; Grahn, B.; Wilcock, B.; Percy, D. Phacoclastic uveitis in the rabbit. Prog. Vet. Comp. Ophthalmol. 1993, 3, 92–97. [Google Scholar]
- Harcourt-Brown, F.M. Textbook of Rabbit Medicine; Butterworth-Heinemann: Oxford, UK, 2002; pp. 52–93. [Google Scholar]
- Giordano, C.; Weigt, A.; Vercelli, A.; Rondena, M.; Grilli, G.; Giudice, C. Immunohistochemical identification of Encephalitozoon cuniculi in phacoclastic uveitis in four rabbits. Vet. Ophthalmol. 2005, 8, 271–275. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Moura, H.; Leitch, J.; Schwartz, D.A. Culture and Propagation of Microsporidia. In The Misrosporidia and Microsporidiosis; Wittner, M., Weiss, L.M., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 363–392. [Google Scholar]
- Mo, L.; Drancourt, M. Monoclonal antibodies for specific detection of Encephalitozoon cuniculi. Clin. Diagn. Lab. Immunol. 2004, 11, 1060–1063. [Google Scholar] [CrossRef]
- Leipig, M.; Matiasek, K.; Rinder, H.; Janik, D.; Emrich, D.; Baiker, K.; Hermanns, W. Value of histopathology, immunohistochemistry, and real-time polymerase chain reaction in the confirmatory diagnosis of Encephalitozoon cuniculi infection in rabbits. J. Vet. Diagn. Investig. 2013, 25, 16–26. [Google Scholar] [CrossRef]
- Shadduck, J.A.; Orenstein, J.M. Comparative pathology of microsporidiosis. Arch. Pathol. Lab. Med. 1993, 117, 1215–1219. [Google Scholar]
- Font, R.L.; Samaha, A.N.; Keener, M.J.; Chevez-Barrios, P.; Goosey, J.D. Corneal microsporidiosis. Report of case, including electron microscopic observations. Ophthalmology 2000, 107, 1769–1775. [Google Scholar] [CrossRef]
- Theng, J.; Chan, C.; Ling, M.L.; Tan, D. Microsporidial keratoconjunctivitis in a healthy contact lens wearer without human immunodeficiency virus infection. Ophthalmology 2001, 108, 976–978. [Google Scholar] [CrossRef]
- Chan, C.M.; Theng, J.T.; Li, L.; Tan, D.T. Microsporidial keratoconjunctivitis in healthy individuals: A case series. Ophthalmology 2003, 110, 1420–1425. [Google Scholar] [CrossRef]
- Kodjikian, L.; Garweg, J.G.; Nguyen, M.; Schaffner, T.; Deplazese, P.; Zimmerli, S. Intraocular microsporidiosis due to Encephalitozoon cuniculi in a patient with idiopathic CD4+ T-lymphocytopenia. Int. J. Med. Microbiol. 2005, 294, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Didier, E.; Ritchie, B.; Greenacre, C.H.; Willis, M.; Martin, C.H. Encephalitozoon cuniculi in the lens of a rabbit with phacoclastic uveitis: Confirmation and treatment. Vet. Comp. Ophthalmol. 1997, 7, 233–238. [Google Scholar]
- Felchle, L.M.; Sigler, R.L. Phacoemulsification for the management of Encephalitozoon cuniculi-induced phacoclastic uveitis in a rabbit. Vet. Ophthalmol. 2002, 5, 211–215. [Google Scholar] [CrossRef]
- Özkan, Ö.; Alcigir, M.E. Subacute Stage of Encephalitozoon cuniculi Infection in Eye Lesions of Rabbit in Turkey. Iran. J. Parasitol. 2018, 13, 301–309. [Google Scholar]
- Poché, R.A.; Hsu, C.W.; McElwee, M.L.; Burns, A.R.; Dickinson, M.E. Macrophages engulf endothelial cell membrane particles preceding pupillary membrane capillary regression. Dev. Biol. 2015, 403, 30–42. [Google Scholar] [CrossRef]
- Zigler, J.S., Jr.; Valapala, M.; Shang, P.; Hose, S.; Goldberg, M.F.; Sinha, D. βA3/A1-crystallin and persistent fetal vasculature (PFV) disease of the eye. Biochem. Biophys. Acta 2016, 1860 Pt B, 287–298. [Google Scholar] [CrossRef]
- Chacaltana, F.D.; Kobashigawa, K.K.; Padua, I.R.; Valdetaro, G.P.; Aldrovani, M.; Laus, J.L. Persistent papillary membrane in Wistar laboratory rats (Rattus Norvegicus, Albinus Variation, Wistar). Cienc. Rural 2017, 47, e20160421. [Google Scholar] [CrossRef] [Green Version]

| Ocular Structure 1 | Weeks after Infection | |||
|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |
| Periocular connective tissue | 3 | 2 | 4 | 4 |
| Extraocular muscles | 2 | 1 | 2 | 3 |
| Sclera | 3 | 3 | 3 | 4 |
| Cornea | 3 | 3 | 4 | 4 |
| Choroidea | 2 | 2 | 3 | 4 |
| Iris | 1 | 1 | 2 | 3 |
| Retina | 1 | 1 | 3 | 3 |
| Lens | 2 | 3 | 3 | 3 |
| No. of rabbits in which E. cuniculi was not detected in any ocular structure | 2 | 1 | 1 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeklová, E.; Levá, L.; Kummer, V.; Jekl, V.; Faldyna, M. Immunohistochemical Detection of Encephalitozoon cuniculi in Ocular Structures of Immunocompetent Rabbits. Animals 2019, 9, 988. https://doi.org/10.3390/ani9110988
Jeklová E, Levá L, Kummer V, Jekl V, Faldyna M. Immunohistochemical Detection of Encephalitozoon cuniculi in Ocular Structures of Immunocompetent Rabbits. Animals. 2019; 9(11):988. https://doi.org/10.3390/ani9110988
Chicago/Turabian StyleJeklová, Edita, Lenka Levá, Vladimír Kummer, Vladimír Jekl, and Martin Faldyna. 2019. "Immunohistochemical Detection of Encephalitozoon cuniculi in Ocular Structures of Immunocompetent Rabbits" Animals 9, no. 11: 988. https://doi.org/10.3390/ani9110988
APA StyleJeklová, E., Levá, L., Kummer, V., Jekl, V., & Faldyna, M. (2019). Immunohistochemical Detection of Encephalitozoon cuniculi in Ocular Structures of Immunocompetent Rabbits. Animals, 9(11), 988. https://doi.org/10.3390/ani9110988