Ancient Microbial Activity in Deep Hydraulically Conductive Fracture Zones within the Forsmark Target Area for Geological Nuclear Waste Disposal, Sweden
Abstract
:1. Introduction
2. Materials and Methods
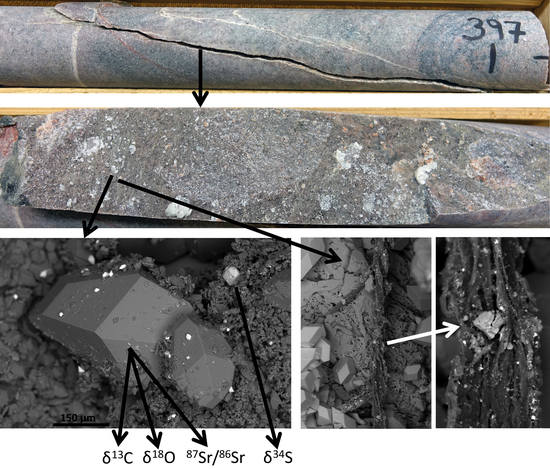
2.1. Materials and Site
2.2. Sample Preparation and Scanning Electron Microscopy
2.3. Secondary Ion Mass Spectrometry (SIMS) for Stable S, O, and C Isotopes
2.4. LA-(MC-)ICP-MS Analyses for 87Sr/86Sr and Rb–Sr Dating
3. Results
3.1. Mineralogy
3.2. Isotope Signatures in Calcite, Waters, and Gases
3.2.1. Carbon Isotopes and Gas Compositions
3.2.2. Oxygen Isotopes
3.2.3. Strontium Isotopes
3.2.4. Sulfur Isotopes
3.2.5. Rb–Sr Dating
3.2.6. Putative Fossilized Microorganisms
4. Discussion
4.1. Episodic Microbial Methanogenesis
4.2. Microbial Anaerobic Oxidation of Methane and Oxidation of Organic Matter
4.3. Microbial Sulfate Reduction
4.4. Putative Microfossils
4.5. Potential Quaternary Mineral Precipitation
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McMahon, S.; Parnell, J. Weighing the deep continental biosphere. FEMS Microbiol. Ecol. 2014, 87, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.C.Y.; Kieft, T.L.; Kuloyo, O.; Linage-Alvarez, B.; van Heerden, E.; Lindsay, M.R.; Magnabosco, C.; Wang, W.; Wiggins, J.B.; Guo, L.; et al. An oligotrophic deep-subsurface community dependent on syntrophy is dominated by sulfur-driven autotrophic denitrifiers. Proc. Natl. Acad. Sci. USA 2016, 113, E7927–E7936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simkus, D.N.; Slater, G.F.; Lollar, B.S.; Wilkie, K.; Kieft, T.L.; Magnabosco, C.; Lau, M.C.Y.; Pullin, M.J.; Hendrickson, S.B.; Wommack, K.E.; et al. Variations in microbial carbon sources and cycling in the deep continental subsurface. Geochim. Cosmochim. Acta 2016, 173, 264–283. [Google Scholar] [CrossRef] [Green Version]
- Chivian, D.; Brodie, E.L.; Alm, E.J.; Culley, D.E.; Dehal, P.S.; DeSantis, T.Z.; Gihring, T.M.; Lapidus, A.; Lin, L.-H.; Lowry, S.R.; et al. Environmental Genomics Reveals a Single-Species Ecosystem Deep Within Earth. Science 2008, 322, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onstott, T.C.; McGown, D.J.; Bakermans, C.; Ruskeeniemi, T.; Ahonen, L.; Telling, J.; Soffientino, B.; Pfiffner, S.M.; Sherwood-Lollar, B.; Frape, S.; et al. Microbial Communities in Subpermafrost Saline Fracture Water at the Lupin Au Mine, Nunavut, Canada. Microb. Ecol. 2009, 58, 786–807. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Holmfeldt, K.; Hubalek, V.; Lundin, D.; Åström, M.; Bertilsson, S.; Dopson, M. Microbial metagenomes from three aquifers in the Fennoscandian shield terrestrial deep biosphere reveal metabolic partitioning among populations. ISME J. 2016, 10, 1192–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, K. Subterranean microbial populations metabolize hydrogen and acetate under in situ conditions in granitic groundwater at 450 m depth in the Aspo Hard Rock Laboratory, Sweden. FEMS Microbiol. Ecol. 2012, 81, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Hallbeck, L.; Pedersen, K. Culture-dependent comparison of microbial diversity in deep granitic groundwater from two sites considered for a Swedish final repository of spent nuclear fuel. FEMS Microbiol. Ecol. 2012, 81, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallbeck, L.; Pedersen, K. Characterization of microbial processes in deep aquifers of the Fennoscandian Shield. Appl. Geochem. 2008, 23, 1796–1819. [Google Scholar] [CrossRef]
- Pedersen, K. Microbial life in granitic rock. FEMS Microbiol. Rev. 1997, 20, 399–414. [Google Scholar] [CrossRef]
- Haveman, S.A.; Pedersen, K.; Ruotsalainen, P. Distribution and metabolic diversity in of microorganisms in deep igneous rock aquifers in Finland. Geomicrobiology 1999, 16, 277–294. [Google Scholar]
- Purkamo, L.; Bomberg, M.; Nyyssönen, M.; Kukkonen, I.; Ahonen, L.; Kietäväinen, R.; Itävaara, M. Dissecting the deep biosphere: Retrieving authentic microbial communities from packer-isolated deep crystalline bedrock fracture zones. FEMS Microbiol. Ecol. 2013, 85, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Bengtsson, A.F.; Edlund, J.S.; Eriksson, L.C. Sulphate-controlled Diversity of Subterranean Microbial Communities over Depth in Deep Groundwater with Opposing Gradients of Sulphate and Methane. Geomicrobiol. J. 2014, 31, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, K. Metabolic activity of subterranean microbial communities in deep granitic groundwater supplemented with methane and H2. ISME J. 2012, 7, 839. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Arlinger, J.; Hallbeck, A.; Hallbeck, L.; Eriksson, S.; Johansson, J. Numbers, biomass and cultivable diversity of microbial populations related to depth and borehole-specific conditions in groundwater from depths of 4 to 450 m in Olkiluoto, Finland. ISME J. 2008, 2, 760–775. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Hernsdorf, A.W.; Konno, U.; Kouduka, M.; Yanagawa, K.; Kato, S.; Sunamura, M.; Hirota, A.; Togo, Y.S.; Ito, K.; et al. Ecological and genomic profiling of anaerobic methane-oxidizing archaea in a deep granitic environment. ISME J. 2017, 12, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Konno, U.; Kouduka, M.; Hirota, A.; Togo, Y.S.; Fukuda, A.; Komatsu, D.; Tsunogai, U.; Tanabe, A.S.; Yamamoto, S.; et al. Deep microbial life in high-quality granitic groundwater from geochemically and geographically distinct underground boreholes. Environ. Microbiol. Rep. 2016, 8, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Nyyssönen, M.; Hultman, J.; Ahonen, L.; Kukkonen, I.; Paulin, L.; Laine, P.; Itävaara, M.; Auvinen, P. Taxonomically and functionally diverse microbial communities in deep crystalline rocks of the Fennoscandian shield. ISME J. 2014, 8, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Sohlberg, E.; Bomberg, M.; Miettinen, H.; Nyyssönen, M.; Salavirta, H.; Vikman, M.; Itävaara, M. Revealing the unexplored fungal communities in deep groundwater of crystalline bedrock fracture zones in Olkiluoto, Finland. Front. Microbiol. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.; Ivarsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubalek, V.; Wu, X.; Eiler, A.; Buck, M.; Heim, C.; Dopson, M.; Bertilsson, S.; Ionescu, D. Connectivity to the surface determines diversity patterns in subsurface aquifers of the Fennoscandian shield. ISME J. 2016, 10, 2447–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapelle, F.H.; O’Neill, K.; Bradley, P.M.; Methe, B.A.; Ciufo, S.A.; Knobel, L.L.; Lovley, D.R. A hydrogen-based subsurface microbial community dominated by methanogens. Nature 2002, 415, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Kietäväinen, R.; Purkamo, L. The origin, source, and cycling of methane in deep crystalline rock biosphere. Front. Microbiol. 2015, 6, 725. [Google Scholar] [CrossRef] [PubMed]
- Onstott, T.C.; Lin, L.H.; Davidson, M.; Mislowack, B.; Borcsik, M.; Hall, J.; Slater, G.; Ward, J.; Lollar, B.S.; Lippmann-Pipke, J.; et al. The Origin and Age of Biogeochemical Trends in Deep Fracture Water of the Witwatersrand Basin, South Africa. Geomicrobiol. J. 2006, 23, 369–414. [Google Scholar] [CrossRef]
- Kotelnikova, S. Microbial production and oxidation of methane in deep subsurface. Earth-Sci. Rev. 2002, 58, 367–395. [Google Scholar] [CrossRef]
- Stevens, T.O.; McKinley, J.P. Lithoautotrophic Microbia, Ecosystems in Deep Basalt Aquifers. Science 1995, 270, 450–454. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Frape, S.K.; Fritz, P.; Macko, S.A.; Welhan, J.A.; Blomqvist, R.; Lahermo, P.W. Evidence for bacterially generated hydrocarbon gas in Canadian shield and fennoscandian shield rocks. Geochim. Cosmochim. Acta 1993, 57, 5073–5085. [Google Scholar] [CrossRef]
- Kieft, T.L.; Walters, C.C.; Higgins, M.B.; Mennito, A.S.; Clewett, C.F.M.; Heuer, V.; Pullin, M.J.; Hendrickson, S.; van Heerden, E.; Sherwood Lollar, B.; et al. Dissolved organic matter compositions in 0.6–3.4 km deep fracture waters, Kaapvaal Craton, South Africa. Org. Geochem. 2018, 118, 116–131. [Google Scholar] [CrossRef]
- Drake, H.; Heim, C.; Roberts, N.M.W.; Zack, T.; Tillberg, M.; Broman, C.; Ivarsson, M.; Whitehouse, M.J.; Åström, M.E. Isotopic evidence for microbial production and consumption of methane in the upper continental crust throughout the Phanerozoic eon. Earth Planet. Sci. Lett. 2017, 470, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Drake, H.; Åström, M.E.; Heim, C.; Broman, C.; Åström, J.; Whitehouse, M.J.; Ivarsson, M.; Siljeström, S.; Sjövall, P. Extreme 13C-depletion of carbonates formed during oxidation of biogenic methane in fractured granite. Nat. Commun. 2015, 6, 7020. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.; Tullborg, E.-L. Paleohydrogeological events recorded by stable isotopes, fluid inclusions and trace elements in fracture minerals in crystalline rock, Simpevarp area, SE Sweden. Appl. Geochem. 2009, 24, 715–732. [Google Scholar] [CrossRef]
- Sandström, B.; Tullborg, E.-L. Episodic fluid migration in the Fennoscandian Shield recorded by stable isotopes, rare earth elements and fluid inclusions in fracture minerals at Forsmark, Sweden. Chem. Geol. 2009, 266, 126–142. [Google Scholar] [CrossRef]
- Drake, H.; Heim, C.; Hogmalm, K.J.; Hansen, B.T. Fracture zone-scale variation of trace elements and stable isotopes in calcite in a crystalline rock setting. Appl. Geochem. 2014, 40, 11–24. [Google Scholar] [CrossRef]
- Heim, C.; Lausmaa, J.; Sjövall, P.; Toporski, J.; Dieing, T.; Simon, K.; Hansen, B.T.; Kronz, A.; Arp, G.; Reitner, J.; et al. Ancient microbial activity recorded in fracture fillings from granitic rocks (Äspö Hard Rock Laboratory, Sweden). Geobiology 2012, 10, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Ziegenbalg, S.B.; Birgel, D.; Hoffmann-Sell, L.; Pierre, C.; Rouchy, J.M.; Peckmann, J. Anaerobic oxidation of methane in hypersaline Messinian environments revealed by 13C-depleted molecular fossils. Chem. Geol. 2012, 292–293, 140–148. [Google Scholar] [CrossRef]
- Peckmann, J.; Thiel, V. Carbon cycling at ancient methane-seeps. Chem. Geol. 2004, 205, 443–467. [Google Scholar] [CrossRef]
- Drake, H.; Åström, M.; Tullborg, E.-L.; Whitehouse, M.J.; Fallick, A.E. Variability of sulphur isotope ratios in pyrite and dissolved sulphate in granitoid fractures down to 1 km depth—Evidence for widespread activity of sulphur reducing bacteria. Geochim. Cosmochim. Acta 2013, 102, 143–161. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P.; Whitehouse, M. Implications of sulfur isotope fractionation in fracture-filling sulfides in crystalline bedrock, Olkiluoto, Finland. Appl. Geochem. 2013, 32, 52–69. [Google Scholar] [CrossRef]
- Sandström, B.; Tullborg, E.-L.; Larson, S.Å.; Page, L. Brittle tectonothermal evolution in the Forsmark area, central Fennoscandian Shield, recorded by paragenesis, orientation and 40Ar/39Ar geochronology of fracture minerals. Tectonophysics 2009, 478, 158–174. [Google Scholar] [CrossRef]
- Andersson, J.; Skagius, K.; Winberg, A.; Lindborg, T.; Ström, A. Site-descriptive modelling for a final repository for spent nuclear fuel in Sweden. Environ. Earth Sci. 2013, 69, 1045–1060. [Google Scholar] [CrossRef]
- Laaksoharju, M.; Smellie, J.; Tullborg, E.-L.; Gimeno, M.; Molinero, J.; Gurban, I.; Hallbeck, L. Hydrogeochemical evaluation and modelling performed within the Swedish site investigation programme. Appl. Geochem. 2008, 23, 1761–1795. [Google Scholar] [CrossRef]
- Sandström, B.; Stephens, M.B. Mineralogy, Geochemistry, Porosity and Redox Properties of Rocks from Forsmark. Compilation of Data from the Regional Model Volume for SR-Site; SKB-R-09-51; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2009. [Google Scholar]
- Stephens, M.B.; Fox, A.; La Pointe, P.; Simeonov, A.; Isaksson, H.; Hermanson, J.; Öhman, J. Geology Forsmark. Site Descriptive Modelling Forsmark Stage 2.2; R-07-45; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2007. [Google Scholar]
- Drake, H.; Sandström, B.; Tullborg, E.-L. Mineralogy and Geochemistry of Rocks and Fracture Fillings from Forsmark and Oskarshamn: Compilation of Data for SR-Can; SKB Report R-06-109; SKB: Stockholm, Sweden, 2006; p. 105. [Google Scholar]
- Saintot, A.; Stephens, M.B.; Viola, G.; Nordgulen, O. Brittle tectonic evolution and paleostress field reconstruction in the southwestern part of the Fennoscandian Shield, Forsmark, Sweden. Tectonics 2011, 30. [Google Scholar] [CrossRef] [Green Version]
- Dahlin, P.; Maskenskaya, O. Boremap Mapping of Core Drilled Borehole KFM24; SKB-Report P-16-28; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2017. [Google Scholar]
- Samuelsson, E.; Rauséus, G. Boremap Mapping of Telescopic Drilled Borehole KFM08D. Forsmark Site Investigation; SKB-Report P-07-103; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2007. [Google Scholar]
- Hurmerinta, E. Forsmark Site Investigations. Difference Flow Logging in Borehole KFM24; SKB-Report P-16-27; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2017. [Google Scholar]
- Teurneau, B.; Forsmark, T.; Forssman, I.; Rhén, I.; Zinn, E. Correlation of Posiva Flow Log Anomalies to Core Mapped Features in KFM01D, KFM07C, KFM08A, KFM08C and KFM10A. Forsmark Site Investigation; SKB-Report P-07-127; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2008. [Google Scholar]
- Laaksoharju, M.; Smellie, J.; Tullborg, E.-L.; Gimeno, M.; Hallbeck, L.; Molinero, J.; Waber, N. Bedrock hydrogeochemistry Forsmark. Site Descriptive Modelling. SDM-Site Forsmark; SKB Report R-08-47; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2008. [Google Scholar]
- Pedersen, K. Microorganisms in Groundwater from Boreholes KFM10A, KFM11A and KFM08D—Numbers, Viability, and Metabolic Diversity. Forsmark Site Investigation; Results from Five Sections 298.0-305.1 m and 478.0-487.5 m in KFM10A, 447.5-454.6 m in KFM11A, and 669.7-676.8 m and 828.4-835.5 m in KFM08D; SKB-Report P-07-198; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2007. [Google Scholar]
- Bergelin, A.; Lindquist, A.; Nilsson, K.; Wacker, P.; Nilsson, A.-C. Hydrochemical Characterisation in Borehole KFM08D. Forsmark Site Investigation; Results from Two Investigated Borehole Sections at 669.7-676.8 m and 828.4-835.5 m; SKB-Report P-07-190; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2007. [Google Scholar]
- Hallbeck, L.; Pedersen, K. Explorative Analysis of Microbes, Colloids and Gases. SDM-Site Forsmark; SKB Report R-08-85; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2008. [Google Scholar]
- Kamber, B.S.; Whitehouse, M.J. Micro-scale sulphur isotope evidence for sulphur cycling in the late Archean shallow ocean. Geobiology 2007, 5, 5–17. [Google Scholar] [CrossRef]
- Drake, H.; Tullborg, E.-L.; Sandberg, B.; Blomfeldt, T.; Åström, M.E. Extreme fractionation and micro-scale variation of sulphur isotopes during bacterial sulphate reduction in Deep groundwater systems. Geochim. Cosmochim. Acta 2015, 161, 1–18. [Google Scholar] [CrossRef]
- Hogmalm, K.J.; Zack, T.; Karlsson, A.K.O.; Sjoqvist, A.S.L.; Garbe-Schonberg, D. In situ Rb-Sr and K-Ca dating by LA-ICP-MS/MS: An evaluation of N2O and SF6 as reaction gases. J. Anal. At. Spectrom. 2017, 32, 305–313. [Google Scholar] [CrossRef]
- Zack, T.; Hogmalm, J. Laser ablation Rb/Sr dating by online chemical separation of Rb and Sr in an oxygen-filled reaction cell. Chem. Geol. 2016, 437, 120–133. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Elburg, M.; Vroon, P.; van der Wagt, B.; Tchalikian, A. Sr and Pb isotopic composition of five USGS glasses (BHVO-2G, BIR-1G, BCR-2G, TB-1G, NKT-1G). Chem. Geol. 2005, 223, 196–207. [Google Scholar] [CrossRef]
- Kiel, S.; Glodny, J.; Birgel, D.; Bulot, L.G.; Campbell, K.A.; Gaillard, C.; Graziano, R.; Kaim, A.; Lazăr, I.; Sandy, M.R.; et al. The Paleoecology, Habitats, and Stratigraphic Range of the Enigmatic Cretaceous Brachiopod Peregrinella. PLoS ONE 2014, 9, e109260. [Google Scholar] [CrossRef] [PubMed]
- Mokadem, F.; Parkinson, I.J.; Hathorne, E.C.; Anand, P.; Allen, J.T.; Burton, K.W. High-precision radiogenic strontium isotope measurements of the modern and glacial ocean: Limits on glacial–interglacial variations in continental weathering. Earth Planet. Sci. Lett. 2015, 415, 111–120. [Google Scholar] [CrossRef]
- Emrich, K.; Ehhalt, D.H.; Vogel, J.C. Carbon isotope fractionation during the precipitation of calcium carbonate. Earth Planet. Sci. Lett. 1970, 8, 363–371. [Google Scholar] [CrossRef]
- Kim, S.-T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Drake, H.; Mathurin, F.A.; Zack, T.; Schäfer, T.; Roberts, N.M.W.; Whitehouse, M.; Karlsson, A.; Broman, C.; Åström, M.E. Incorporation of metals into calcite in a deep anoxic granite aquifer. Environ. Sci. Technol. 2018, 52, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Boehme, S.E.; Blair, N.E.; Chanton, J.P.; Martens, C.S. A mass balance of 13C and 12C in an organic-rich methane-producing marine sediment. Geochim. Cosmochim. Acta 1996, 60, 3835–3848. [Google Scholar] [CrossRef]
- Irwin, H.; Cultis, C.; Coleman, M. Isotopic evidence for source of diagenetic carbonates formed during burial of organic-rich sediments. Nature 1977, 269, 209–213. [Google Scholar] [CrossRef]
- Budai, J.M.; Martini, A.M.; Walter, L.M.; Ku, T.C.W. Fracture-fill calcite as a record of microbial methanogenesis and fluid migration; a case study from the Devonian Antrim Shale, Michigan Basin. Geofluids 2002, 2, 163–183. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P.; Whitehouse, M. Biogenic processes in crystalline bedrock fractures indicated by carbon isotope signatures of secondary calcite. Appl. Geochem. 2016, 67, 30–41. [Google Scholar] [CrossRef]
- Sahlstedt, E.; Karhu, J.A.; Pitkänen, P. Indications for the past redox environments in deep groundwaters from the isotopic composition of carbon and oxygen in fracture calcite, Olkiluoto, SW Finland. Isotopes Environ. Health Stud. 2010, 46, 370–391. [Google Scholar] [CrossRef] [PubMed]
- Clauer, N.; Frape, S.K.; Fritz, B. Calcite veins of the Stripa granite (Sweden) as records of the origin of the groundwaters and their interactions with the granitic body. Geochim. Cosmochim. Acta 1989, 53, 1777–1781. [Google Scholar] [CrossRef]
- Kietäväinen, R.; Ahonen, L.; Niinikoski, P.; Nykänen, H.; Kukkonen, I.T. Abiotic and biotic controls on methane formation down to 2.5 km depth within the Precambrian Fennoscandian Shield. Geochim. Cosmochim. Acta 2017, 202, 124–145. [Google Scholar] [CrossRef]
- Etiope, G.; Sherwood Lollar, B. Abiotic methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Lovley, D.R.; Klug, M.J. Sulfate Reducers Can Outcompete Methanogens at Freshwater Sulfate Concentrations. Appl. Environ. Microbiol. 1983, 45, 187–192. [Google Scholar] [PubMed]
- Sela-Adler, M.; Ronen, Z.; Herut, B.; Antler, G.; Vigderovich, H.; Eckert, W.; Sivan, O. Co-existence of Methanogenesis and Sulfate Reduction with Common Substrates in Sulfate-Rich Estuarine Sediments. Front. Microbiol. 2017, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Holmer, M.; Kristensen, E. Coexistence of sulfate reduction and methane production in an organic-rich sediment. Mar. Ecol. Prog. Ser. 1994, 107, 177–184. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Parkes, R.J. Role of sulfate reduction and methane production by organic carbon degradation in eutrophic fjord sediments (Limfjorden, Denmark). Limnol. Oceanogr. 2010, 55, 1338–1352. [Google Scholar] [CrossRef] [Green Version]
- Bernard, B.B.; Brooks, J.M.; Sackett, W.M. Light hydrocarbons in recent Texas continental shelf and slope sediments. J. Geophys. Res. Oceans 1978, 83, 4053–4061. [Google Scholar] [CrossRef]
- Etiope, G.; Schoell, M. Abiotic Gas: Atypical, But Not Rare. Elements 2014, 10, 291–296. [Google Scholar] [CrossRef]
- Garcia, J.-L.; Patel, B.K.C.; Ollivier, B. Taxonomic, Phylogenetic, and Ecological Diversity of Methanogenic Archaea. Anaerobe 2000, 6, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M.J.; Faber, E.; Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim. Cosmochim. Acta 1986, 50, 693–709. [Google Scholar] [CrossRef]
- Sandström, B.; Tullborg, E.-L.; de Torres, T.; Ortiz, J.E. The occurrence and potential origin of asphaltite in bedrock fractures, Forsmark, central Sweden. GFF 2006, 128, 234–242. [Google Scholar] [CrossRef]
- Knittel, K.; Boetius, A. Anaerobic Oxidation of Methane: Progress with an Unknown Process. Ann. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, W.; Seifert, R.; Nauhaus, K.; Treude, T.; Thiel, V.; Blumenberg, M.; Knittel, K.; Gieseke, A.; Peterknecht, K.; Pape, T.; et al. Microbial reefs in the Black Sea fueled by anaerobic oxidation of methane. Science 2002, 297, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Orphan, V.J.; Hinrichs, K.U.; Ussler, W.; Paull, C.K.; Taylor, L.T.; Sylva, S.P.; Hayes, J.M.; Delong, E.F. Comparative Analysis of Methane-Oxidizing Archaea and Sulfate-Reducing Bacteria in Anoxic Marine Sediments. Appl. Environ. Microbiol. 2001, 67, 1922–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomberg, M.; Nyyssönen, M.; Pitkänen, P.; Lehtinen, A.; Itävaara, M. Active Microbial Communities Inhabit Sulphate-Methane Interphase in Deep Bedrock Fracture Fluids in Olkiluoto, Finland. BioMed Res. Int. 2015, 2015, 979530. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, M.; Birgel, D.; Dela Pierre, F.; Martire, L.; Clari, P.; Spötl, C.; Peckmann, J. Polyphasic carbonate precipitation in the shallow subsurface: Insights from microbially-formed authigenic carbonate beds in upper Miocene sediments of the Tertiary Piedmont Basin (NW Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 329–330, 158–172. [Google Scholar] [CrossRef]
- Feng, D.; Chen, D.; Peckmann, J.; Bohrmann, G. Authigenic carbonates from methane seeps of the northern Congo fan: Microbial formation mechanism. Mar. Pet. Geol. 2010, 27, 748–756. [Google Scholar] [CrossRef]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M.J.; Faber, E. Methane oxidation in sediment and water column environments—Isotope evidence. Org. Geochem. 1986, 10, 759–768. [Google Scholar] [CrossRef]
- Campbell, K.A.; Farmer, J.D.; des Marais, D. Ancient hydrocarbon seeps from the Mesozoic convergent margin of California: Carbonate geochemistry, fluids and palaeoenvironments. Geofluids 2002, 2, 63–94. [Google Scholar] [CrossRef]
- Peckmann, J.; Goedert, J.L.; Thiel, V.; Michaelis, W.; Reitner, J. A comprehensive approach to the study of methane-seep deposits from the Lincoln Creek Formation, western Washington State, USA. Sedimentology 2002, 49, 855–873. [Google Scholar] [CrossRef]
- Roberts, H.H.; Aharon, P. Hydrocarbon-derived carbonate buildups of the northern Gulf of Mexico continental slope: A review of submersible investigations. Geo-Mar. Lett. 1994, 14, 135–148. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Smock, A.M.; Cypionka, H. Sulfur isotope fractionation during experimental precipitation of iron(II) and manganese(II) sulfide at room temperature. Chem. Geol. 1998, 146, 127–134. [Google Scholar] [CrossRef]
- Sim, M.S.; Bosak, T.; Ono, S. Large sulfur isotope fractionation does not require disproportionation. Science 2011, 333, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Kiyosu, Y.; Krouse, H.R. The role of organic acid in the abiogenic reduction of sulfate and the sulfur isotope effect. Geochem. J. 1990, 24, 21–27. [Google Scholar] [CrossRef]
- Kohn, M.J.; Riciputi, L.R.; Stakes, D.; Orange, D.L. Sulfur isotope variability in biogenic pyrite; reflections of heterogeneous bacterial colonization? Am. Mineral. 1998, 83, 1454–1468. [Google Scholar] [CrossRef]
- Borowski, W.S.; Rodriguez, N.M.; Paull, C.K.; Ussler, W., III. Are 34S-enriched authigenic sulfide minerals a proxy for elevated methane flux and gas hydrates in the geologic record? Mar. Pet. Geol. 2013, 43, 381–395. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Böttcher, M.E.; Lüschen, H.; Neretin, L.N.; Volkov, I.I. Anaerobic methane oxidation and a deep H2S sink generate isotopically heavy sulfides in Black Sea sediments 1. Geochim. Cosmochim. Acta 2004, 68, 2095–2118. [Google Scholar] [CrossRef]
- Drake, H.; Hallbeck, L.; Pedersen, K.; Rosdahl, A.; Tullborg, E.-L.; Wallin, B.; Sandberg, B.; Blomfeldt, T. Investigation of Sulphide Production in Core-Drilled Boreholes in Äspö Hard Rock Laboratory. Boreholes KA3110A, KA3385A and KA3105A; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2013. [Google Scholar]
- Tullborg, E.-L.; Smellie, J.; Nilsson, A.-C.; Gimeno, M.J.; Auqué, L.F.; Brüchert, V.; Molinero, J. SR-Site—Sulphide Content in the Groundwater at Forsmark; SKB Report TR-10-39; Swedish Nuclear Fuel and Waste Management Co. (SKB): Stockholm, Sweden, 2010. [Google Scholar]
- Ivarsson, M.; Bengtson, S.; Drake, H.; Francis, W. Fungi in Deep Subsurface Environments. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Bengtson, S.; Rasmussen, B.; Ivarsson, M.; Muhling, J.; Broman, C.; Marone, F.; Stampanoni, M.; Bekker, A. Fungus-like mycelial fossils in 2.4-billion-year-old vesicular basalt. Nat. Ecol. Evol. 2017, 1, 0141. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, M.; Holm, N.G.; Neubeck, A. The Deep Biosphere of the Subseafloor Igneous Crust. In Trace Metal Biogeochemistry and Ecology of Deep-Sea Hydrothermal Vent Systems; Demina, L.L., Galkin, V.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 143–166. [Google Scholar]
- Drake, H.; Ivarsson, M. The role of anaerobic fungi in fundamental biogeochemical cycles in the deep biosphere. Fungal Biol. Rev. 2018, 32, 20–25. [Google Scholar] [CrossRef]
- Ivarsson, M.; Schnürer, A.; Bengtson, S.; Neubeck, A. Anaerobic fungi: A potential source of biological H2 in the oceanic crust. Front. Microbiol. 2016, 7, 674. [Google Scholar] [CrossRef] [PubMed]
- Parkes, R.J.; Linnane, C.D.; Webster, G.; Sass, H.; Weightman, A.J.; Hornibrook, E.R.C.; Horsfield, B. Prokaryotes stimulate mineral H2 formation for the deep biosphere and subsequent thermogenic activity. Geology 2011, 39, 219–222. [Google Scholar] [CrossRef]








| Borehole | Sample | Vertical Depth | δ13Cmin | δ13Cmax | nδ13C | δ18Omin | δ18Omax | nδ18O | 87Sr/86Srmin | ± | 87Sr/86Srmax | ± | n87Sr/86Sr | δ34Smin | δ34Smax | nδ34S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (m) | ‰, V-PDB | ‰, V-PDB | ‰, V-PDB | ‰, V-PDB | ‰, V-CDT | ‰, V-CDT | ||||||||||
| KFM01D | 316 | −253.27 | −7.4 | −2.8 | 15 | −12.7 | −5.9 | 15 | 0.7106 | 0.0016 | 0.7118 | 0.0012 | 2 | 0 | ||
| KFM01D | 431 | −341.50 | −10.9 | −4.5 | 13 | −13.4 | −6.2 | 18 | 0.7054 | 0.0025 | 1 | 0 | ||||
| KFM02A | 162 | −155.29 | 0.0 | 11.3 | 10 | 0 | 0 | 0 | ||||||||
| KFM02A | 175 | −167.94 | −31.1 | −8.7 | 10 | −12.9 | −10.3 | 7 | 0.7142 | 0.0010 | 1 | 0 | ||||
| KFM08D | 677 | −544.25 | −12.6 | −0.5 | 21 | −14.5 | −8.0 | 21 | 0.7097 | 0.0011 | 0.7124 | 0.0012 | 10 | 0 | ||
| KFM08D | 678 | −544.48 | −8.9 | −2.9 | 25 | −8.0 | −17.9 | 18 | 0 | 0 | ||||||
| KFM08D | 830 | −662.86 | −4.5 | −4.2 | 3 | −16.1 | −8.3 | 5 | 0 | −3.5 | −1.3 | 13 | ||||
| KFM08D | 832 | −664.86 | 0 | 0 | 0 | 0.0 | 12.4 | 8 | ||||||||
| KFM24 | 193 | −191.00 | −9.1 | 18.5 | 4 | −13.1 | −8.2 | 7 | 0 | 0 | ||||||
| KFM24 | 194 | −192.00 | −16.1 | −4.9 | 13 | −16.1 | −7.4 | 21 | 0.7182 | 0.0012 | 1 | 0 | ||||
| KFM24 | 379 | −376.00 | −17.1 | 20.2 | 30 | −13.1 | −7.9 | 16 | 0.7176 | 0.0033 | 1 | −11.7 | 37.8 | 24 | ||
| KFM24 | 396 | −392.00 | −36.2 | −10.4 | 24 | −13.7 | −9.8 | 11 | 0.7143 | 0.0016 | 0.7159 | 0.0023 | 2 | −1.3 | 36.0 | 29 |
| KFM24 | 398 | −394.00 | −32.6 | −3.0 | 13 | −13.9 | −8.6 | 13 | 0.7114 | 0.0031 | 1 | −2.4 | 21.2 | 27 | ||
| KFM24 | 399 | −395.00 | −35.8 | −10.6 | 23 | −12.4 | −7.1 | 16 | 0.7089 | 0.0031 | 0.7161 | 0.0025 | 4 | −0.7 | 31.0 | 26 |
| KFM24 | 410 | −406.00 | −29.3 | 17.6 | 17 | −14.7 | −7.0 | 19 | 0.7150 | 0.0033 | 0.7197 | 0.0024 | 3 | 0 | ||
| KFM24 | 413 | −409.53 | −29.8 | −9.9 | 16 | −15.0 | −6.2 | 18 | 0.7184 | 0.0034 | 1 | −11.5 | 29.0 | 25 | ||
| KFM24 | 414 | −410.53 | −17.4 | −12.0 | 15 | −14.8 | −12.1 | 15 | 0.7105 | 0.0029 | 0.71603 | 0.00076 | 3 | 7.1 | 28.4 | 27 |
| Total | −36.2 | 20.2 | 252 | −16.1 | −5.9 | 220 | 0.7054 | 0.0025 | 0.7197 | 0.0024 | 30 | −11.7 | 37.8 | 179 |
| Borehole | Sampling Date | Upper Packer | Lower Packer | Na | K | Ca | Mg | HCO3 | Cl | SO4 | Fe | Mn | Sr | DOC | pH (lab) | S2 in HS | δ13C | δ34SSO4 | 87Sr/86Sr | δ2H | δ18O | Temp |
| Groundwaters | (m) | (m) | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | ‰, V-PDB | ‰, V-CDT | ‰ V-SMOW | ‰ V-SMOW | °C | |||
| KFM01D | 2015 | 312.50 | 319.61 | 1790 | 17.4 | 1080 | 90.9 | 75.9 | 4466 | 273 | 0.804 | 0.344 | 11.4 | 3.3 | 7.73 | 0.29 | −11.40 | 28.9 | 0.720596 | −70.8 | −10.01 | |
| KFM01D | 2006 | 428.50 | 435.64 | 1550 | 9.02 | 1430 | 19.5 | 35.8 | 4940 | 125 | 1.93 | 0.182 | 17.4 | 3.7 | 7.55 | 0.01 | −13.20 | 26.7 | 0.720149 | −74.9 | −10.70 | |
| KFM01D | 2015 | 428.50 | 435.64 | 1750 | 7.20 | 1610 | 18.4 | 14.7 | 5272 | 67.2 | 0.0437 | 0.0772 | 19.1 | 4.6 | 7.81 | 3.22 | 42.9 | 0.720040 | −72.6 | −11.00 | ||
| KFM08D | 2007 | 669.70 | 676.84 | 1900 | 5.42 | 2740 | 4.9 | 6.98 | 7460 | 101 | 0.0044 | 0.0498 | 29.3 | b.d. | 8.14 | b.d. | 31.6 | 0.717523 | −84.0 | −12.30 | ||
| KFM08D | 2013 | 669.70 | 676.84 | 1780 | 7.23 | 2080 | 10.4 | 10.0 | 6455 | 75.4 | b.d. | 0.114 | 22.8 | 0.7 | 8.40 | b.d. | −4.50 | 36.6 | 0.718009 | −84.1 | −13.11 | |
| KFM08D | 2007 | 828.40 | 835.54 | 1990 | 6.26 | 2960 | 8.5 | 9.07 | 8160 | 156 | 0.114 | 0.0725 | 33.1 | b.d. | 7.93 | 0.07 | 26.6 | 0.717524 | −82.5 | −11.60 | ||
| KFM24 | 2016 | 191.50 | 198.62 | 817 | 4.79 | 411 | 11.3 | 21.0 | 1965 | 44.0 | 0.789 | 0.119 | 4.5 | 9.3 | 7.20 | 0.17 | −15.90 | 33.2 | 0.719201 | −107.8 | −14.40 | 15.7 |
| KFM24 | 2016 | 395.50 | 402.62 | 1500 | 3.08 | 1740 | -0.5 | 6.80 | 5148 | 15.1 | b.d. | 0.00397 | 20.0 | 2.0 | 8.30 | b.d. | 48.5 * | 0.719778 * | −93.4 | −13.09 | 16.1 | |
| KFM24 | 2016(nov) | 410.00 | 417.12 | 1600 | 3.27 | 1930 | -0.5 | 6.50 | 5564 | 8.94 | 0.0049 | 0.00071 | 22.4 | 2.0 | 8.83 | 0.29 | 57.5 | 0.719968 | −91.8 | −13.41 | 15.9 | |
| KFM24 | 2016(dec) ** | 410.00 | 417.12 | 1460 | 3.60 | 1730 | 1.2 | 9.60 | 5032 | 15.8 | b.d. | 0.0066 | 19.9 | 14.7 | 8.83 | 0.14 | −21.90 | 37.6 | 0.719916 | −91.5 | −3.21 | 15.4 |
| Borehole | Sampling Date | Upper Packer | Lower Packer | He | Ar | N2 | CO2 | O2 | CH4 | H2 | C2H2 | C2H4 | C2H6 | C3H4 | C3H6 | C3H8 | C1/(C2+C3) | |||||
| Gases, Concentrations | (m) | (m) | (mL/L) | (mL/L) | (mL/L) | (mL/L) | (mL/L) | (mL/L) | (uL/L) | (uL/L) | (uL/L) | (uL/L) | (uL/L) | (uL/L) | (uL/L) | |||||||
| KFM01D * | 2015 | 312.50 | 319.61 | 11 | b.d. | 133 | 0.32 | b.d. | 0.64 | 3.5 | b.d. | b.d. | 0.28 | b.d. | b.d. | b.d. | 2289 | |||||
| KFM01D | 2006 | 428.50 | 435.64 | 26 | 1.10 | 64 | 0.20 | 0.062 | 0.14 | b.d. | b.d. | 0.09 | 2.5 | b.d. | b.d. | 0.57 | 54 | |||||
| KFM01D | 2015 | 428.50 | 435.64 | 23 | 1.30 | 91 | 0.12 | 8.0 | 0.41 | b.d. | b.d. | b.d. | 3.0 | b.d. | b.d. | 0.62 | 137 | |||||
| KFM08D | 2007 | 669.70 | 676.84 | 23 | 1.70 | 73 | 0.020 | 0.035 | 0.090 | b.d. | b.d. | 0.10 | 0.49 | b.d. | b.d. | 0.20 | 153 | |||||
| KFM08D | 2013 | 669.70 | 676.84 | 22 | 1.20 | 77 | 0.040 | 0.25 | 0.10 | b.d. | 1.10 | 1.40 | 1.4 | b.d. | b.d. | 0.75 | 26 | |||||
| KFM08D | 2007 | 828.40 | 835.54 | 24 | 1.40 | 85 | 0.030 | b.d. | 0.060 | b.d. | b.d. | 0.11 | 0.55 | b.d. | b.d. | 0.22 | 91 | |||||
| KFM24 | 2016 | 395.50 | 402.62 | 10 | 1.30 | 93 | 0.019 | 1.4 | 0.19 | b.d. | b.d. | b.d. | 1.1 | b.d. | b.d. | b.d. | 173 | |||||
| KFM24 | 2016 | 410.00 | 417.12 | 10 | 1.30 | 121 | 0.018 | 3.7 | 0.18 | b.d. | b.d. | b.d. | 1.1 | b.d. | b.d. | b.d. | 164 | |||||
| Borehole | Sampling Date | Upper Packer | Lower Packer | δ2HCH4 | δ13CCO2 | δ13CCH4 | δ18OCO2 | |||||||||||||||
| Gases, Isotopes | (m) | (m) | ‰ V-SMOW | ± ‰, 1s | ‰, V-PDB | ± ‰, 1s | ‰, V-PDB | ± ‰, 1s | ‰ V-SMOW | ± ‰, 1s | ||||||||||||
| KFM24 | 2016 | 410.00 | 417.12 | −227 | 10.0 | −12.5 | 0.3 | −49.3 | 1.5 | 36.1 | 0.2 | |||||||||||
| KFM24 | 2016 *** | 410.00 | 417.12 | −242 | 10.0 | −13.4 | 0.3 | −50.4 | 1.5 | 35.4 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drake, H.; Ivarsson, M.; Tillberg, M.; Whitehouse, M.J.; Kooijman, E. Ancient Microbial Activity in Deep Hydraulically Conductive Fracture Zones within the Forsmark Target Area for Geological Nuclear Waste Disposal, Sweden. Geosciences 2018, 8, 211. https://doi.org/10.3390/geosciences8060211
Drake H, Ivarsson M, Tillberg M, Whitehouse MJ, Kooijman E. Ancient Microbial Activity in Deep Hydraulically Conductive Fracture Zones within the Forsmark Target Area for Geological Nuclear Waste Disposal, Sweden. Geosciences. 2018; 8(6):211. https://doi.org/10.3390/geosciences8060211
Chicago/Turabian StyleDrake, Henrik, Magnus Ivarsson, Mikael Tillberg, Martin J. Whitehouse, and Ellen Kooijman. 2018. "Ancient Microbial Activity in Deep Hydraulically Conductive Fracture Zones within the Forsmark Target Area for Geological Nuclear Waste Disposal, Sweden" Geosciences 8, no. 6: 211. https://doi.org/10.3390/geosciences8060211
APA StyleDrake, H., Ivarsson, M., Tillberg, M., Whitehouse, M. J., & Kooijman, E. (2018). Ancient Microbial Activity in Deep Hydraulically Conductive Fracture Zones within the Forsmark Target Area for Geological Nuclear Waste Disposal, Sweden. Geosciences, 8(6), 211. https://doi.org/10.3390/geosciences8060211