Oxidative Stress in Patients with Drug Resistant Partial Complex Seizure
Abstract
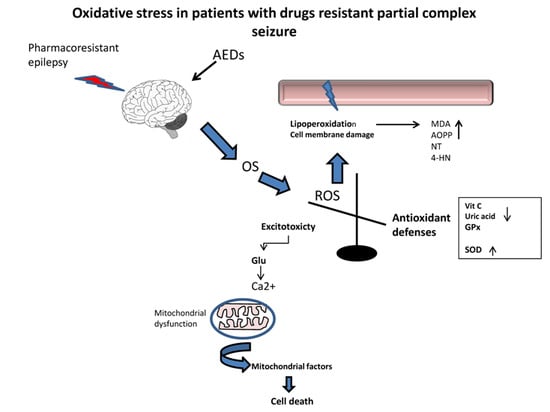
:1. Introduction
2. Materials and Methods
2.1. Patient Information
2.2. Samples
2.3. Determination of Lipid Peroxidation
2.4. AOPP Assay
2.5. Advanced Glycation End Products AGEs
2.6. Nitric Oxide
2.7. Western Blot Analysis for 3-Nitrotyrosine and 4-Hidroxynonenal
2.8. SOD Activity
2.9. Vitamin C
2.10. Uric Acid
2.11. Gluthatione
2.12. Ethical Considerations
2.13. Statistical Processing
3. Results
3.1. Proteins and Lipid Damage and Advanced Glycation
3.2. Nitric Oxide
3.3. Expression of 4-Hydroxy-2-Noneal and 3-Nitrotyrosine
3.4. Antioxidant Evaluation
3.5. Correlation of Oxidative Stress Parameters with Clinical Data
4. Discussion
4.1. Proteins, Lipid Damage and Advanced Glycation Products
4.2. Antioxidants
4.3. Correlation of Oxidative Stress Parameters with Clinical Data
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardenas-Rodriguez, N.; Huerta-Gertrudis, B.; Rivera-Espinosa, L.; Montesinos-Correa, H.; Bandala, C.; Carmona-Aparicio, L.; Coballase-Urrutia, E. Role of oxidative stress in refractory epilepsy: Evidence in patients and experimental models. Int. J. Mol. Sci. 2013, 14, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.A.; Annegers, J.F.; Kurland, L.T. Prevalence of epilepsy in rochester, minnesota: 1940–1980. Epilepsia 1991, 32, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Regesta, G.; Tanganelli, P. Clinical aspects and biological bases of drug resistant epilepsies. Epilepsy Res. 1999, 34, 109–122. [Google Scholar] [CrossRef]
- Henshall, D.C.; Meldrum, B.S.; Soman, S.; Korah, P.K.; Jayanarayanan, S.; Mathew, J.; Paulose, C.S. Cell death and survival mechanisms after single and repeated brief seizures oxidative stress induced nmda receptor alteration leads to spatial memory deficits in temporal lobe epilepsy: Ameliorative effects of withania somnifera and withanolide a. Neurochem. Res. 2012, 37, 1915–1927. [Google Scholar]
- Fujikawa, D.G.; Shinmei, S.S.; Cai, B. Kainic acid-induced seizures produce necrotic, not apoptotic, neurons with internucleosomal DNA cleavage: Implications for programmed cell death mechanisms. Neuroscience 2000, 98, 41–53. [Google Scholar] [CrossRef]
- Aguiar, C.C.; Almeida, A.B.; Araujo, P.V.; de Abreu, R.N.; Chaves, E.M.; do Vale, O.C.; Macedo, D.S.; Woods, D.J.; Fonteles, M.M.; Vasconcelos, S.M. Oxidative stress and epilepsy: Literature review. Oxid. Med. Cell. Longev. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sudha, K.; Rao, A.V.; Rao, A. Oxidative stress and antioxidants in epilepsy. Clin. Chim. Acta 2001, 303, 19–24. [Google Scholar] [CrossRef]
- Waldbaum, S.; Patel, M. Mitochondrial dysfunction and oxidative stress: A contributing link to acquired epilepsy? J. Bioenerg. Biomembr. 2010, 42, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Natrella, F.; Belmonte, G.; Miracco, C.; Cervellati, F.; Ciccoli, L.; Mariottini, A.; Rocchi, R.; Vatti, G.; Bua, A.; et al. Nadph oxidase activation and 4-hydroxy-2-nonenal/aquaporin-4 adducts as possible new players in oxidative neuronal damage presents in drug-resistant epilepsy. Biochim. Biophys. Acta 2015, 1852, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E.; Kyllerman, M.; Marklund, S. Superoxide dismutase and glutathione peroxidase function in progressive myoclonus epilepsies. Epilepsy Res. 2000, 40, 33–39. [Google Scholar] [CrossRef]
- Lopez, J.; Gonzalez, M.E.; Lorigados, L.; Morales, L.; Riveron, G.; Bauza, J.Y. Oxidative stress markers in surgically treated patients with refractory epilepsy. Clin. Biochem. 2007, 40, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.H.; Lin, Y.T.; Wu, C.W.; Chao, Y.M.; Chang, A.Y.; Chan, J.Y. Peripheral inflammation increases seizure susceptibility via the induction of neuroinflammation and oxidative stress in the hippocampus. J. Biomed. Sci. 2015, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Smith, J.N.; Patel, M. Metabolic dysfunction and oxidative stress in epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Chen, S.D.; Lin, T.K.; Liou, C.W.; Chang, W.N.; Chan, S.H.; Chang, A.Y. Upregulation of nitric oxide synthase ii contributes to apoptotic cell death in the hippocampal ca3 subfield via a cytochrome c/caspase-3 signaling cascade following induction of experimental temporal lobe status epilepticus in the rat. Neuropharmacology 2007, 52, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Chen, S.D.; Liou, C.W.; Lin, T.K.; Chang, W.N.; Chan, S.H.; Chang, A.Y. Contribution of nitric oxide, superoxide anion, and peroxynitrite to activation of mitochondrial apoptotic signaling in hippocampal ca3 subfield following experimental temporal lobe status epilepticus. Epilepsia 2009, 50, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Armenta, M.; Nava-Ruiz, C.; Juarez-Rebollar, D.; Rodriguez-Martinez, E.; Gomez, P.Y. Oxidative stress associated with neuronal apoptosis in experimental models of epilepsy. Oxid. Med. Cell. Longev. 2014, 2014, 293689. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Jeong, J.H.; Chung, Y.H.; Kim, W.K.; Ko, K.H.; Bach, J.H.; Hong, J.S.; Yoneda, Y.; Kim, H.C. Role of oxidative stress in epileptic seizures. Neurochem. Int. 2011, 59, 122–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorigados, P.L.; Orozco Suárez, S.; Morales, C.L.; Garcia, M.I.; Estupinan, D.B.; Bender del Busto, J.E.; Pavon, F.N.; Paula, P.B.; Rocha, A.L. Neuronal death in the neocortex of drug resistant temporal lobe epilepsy patients. Neurologia 2008, 23, 555–565. [Google Scholar]
- Lorigados, L.; Orozco Suárez, S.; Morales-Chacon, L.; Estupiñán, B.; García, I.; Rocha, L. Excitotoxicity and neuronal death in epilepsy. Biotecnol. Apl. 2013, 30, 9–16. [Google Scholar]
- Wade, C.R.; van Rij, A.M. Plasma thiobarbituric acid reactivity: Reaction conditions and the role of iron, antioxidants and lipid peroxy radicals on the quantitation of plasma lipid peroxides. Life Sci. 1988, 43, 1085–1093. [Google Scholar] [CrossRef]
- Witko, V.; Nguyen, A.T.; Descamps-Latscha, B. Microtiter plate assay for phagocyte-derived taurine-chloramines. J. Clin. Lab. Anal. 1992, 6, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.L. Analysis of extracellular superoxide dismutase in tissue homogenates and extracellular fluids. Methods Enzymol. 1990, 186, 260–265. [Google Scholar] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin e. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Carlson, C.; Dugan, P.; Kirsch, H.E.; Friedman, D. Sex differences in seizure types and symptoms. Epilepsy Behav. 2014, 41, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunelli, E.; Domanico, F.; La Russa, D.; Pellegrino, D. Sex differences in oxidative stress biomarkers. Curr. Drug Targets 2014, 15, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Henshall, D.C. Apoptosis signalling pathways in seizure-induced neuronal death and epilepsy. Biochem. Soc. Trans. 2007, 35, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Friedman, A.; Dingledine, R.J. The role of inflammation in epileptogenesis. Neuropharmacology 2013, 69, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezzani, A.; Maroso, M.; Balosso, S.; Sanchez, M.A.; Bartfai, T. Il-1 receptor/toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav. Immun. 2011, 25, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Balosso, S.; Ravizza, T. Inflammation and epilepsy. Handb. Clin. Neurol. 2012, 107, 163–175. [Google Scholar] [PubMed] [Green Version]
- Castellanos Ortega, M.R.; Cruz, A.R.; Lorigados, P.L.; De La Cuetara, B.K. Purification and characterization of murine beta-nerve growth factor. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 245–252. [Google Scholar] [CrossRef]
- Lorigados, P.L.; Morales Chacon, L.M.; Orozco, S.S.; Pavon, F.N.; Estupinan, D.B.; Serrano, S.T.; Garcia, M.I.; Rocha, A.L. Inflammatory mediators in epilepsy. Curr. Pharm. Des. 2013, 19, 6766–6772. [Google Scholar] [CrossRef]
- Ureña-Guerrero, M.E.; Feria-Velasco, A.; Gudiño-Cabrera, G.; Camin-Espuny, A.; Beas-Zrate, C. Modifications in the seizures susceptibility by excitotoxic neuronal damage and possible relationship with the pharmacoresistance. In Pharmacoresistance in Eplepsy. From Genes and Molecules to Promising Therapy; Rocha, A.L., Cavalheiro, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 59–76. [Google Scholar]
- Yis, U.; Seckin, E.; Kurul, S.H.; Kuralay, F.; Dirik, E. Effects of epilepsy and valproic acid on oxidant status in children with idiopathic epilepsy. Epilepsy Res. 2009, 84, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Turkdogan, D.; Toplan, S.; Karakoc, Y. Lipid peroxidation and antioxidative enzyme activities in childhood epilepsy. J. Child Neurol. 2002, 17, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Basciani, F.; Trotta, D.; Pomilio, M.P.; Morgese, G.; Chiarelli, F. Serum copper, zinc, selenium, glutathione peroxidase and superoxide dismutase levels in epileptic children before and after 1 year of sodium valproate and carbamazepine therapy. Epilepsy Res. 2002, 48, 71–75. [Google Scholar] [CrossRef]
- Gunes, S.; Dirik, E.; Yis, U.; Seckin, E.; Kuralay, F.; Kose, S.; Unalp, A. Oxidant status in children after febrile seizures. Pediatr. Neurol. 2009, 40, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; de Faria, J.B. Which comes first: Renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic. Res. 2007, 41, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of tnf. Oxid. Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflamm. 2011, 2011, 514623. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Smith, C. Ageing-associated oxidative stress and inflammation are alleviated by products from grapes. Oxid. Med. Cell. Longev. 2016, 2016, 6236309. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; van Horssen, J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim. Biophys. Acta 2016, 1862, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Flohe, L.; Brigelius-Flohe, R.; Saliou, C.; Traber, M.G.; Packer, L. Redox regulation of nf-kappa b activation. Free Radic. Biol. Med. 1997, 22, 1115–1126. [Google Scholar] [CrossRef]
- Lorigados Pedre, L.; Morales Chacon, L.M.; Pavon Fuentes, N.; Robinson Agramonte, M.L.A.; Serrano Sanchez, T.; Cruz-Xenes, R.M.; Diaz Hung, M.L.; Estupinan Diaz, B.; Baez Martin, M.M.; Orozco Suárez, S. Follow-up of peripheral il-1beta and il-6 and relation with apoptotic death in drug-resistant temporal lobe epilepsy patients submitted to surgery. Behav. Sci. (Basel, Switzerland) 2018, 8, 21. [Google Scholar]
- Keskin Guler, S.; Aytac, B.; Durak, Z.E.; Gokce Cokal, B.; Gunes, N.; Durak, I.; Yoldas, T. Antioxidative-oxidative balance in epilepsy patients on antiepileptic therapy: A prospective case-control study. Neurol. Sci. 2016, 37, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Donmezdil, N.; Cevik, M.U.; Ozdemir, H.H.; Tasin, M. Investigation of pon1 activity and mda levels in patients with epilepsy not receiving antiepileptic treatment. Neuropsychiatr. Dis. Treat. 2016, 12, 1013–1017. [Google Scholar] [PubMed]
- Yehuda, S.; Rabinovitz, S.; Carasso, R.L.; Mostofsky, D.I. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol. Aging 2002, 23, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.A.; Horrocks, L.A. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell. Mol. Neurobiol. 1998, 18, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Leonarduzzi, G.; Arkan, M.C.; Basaga, H.; Chiarpotto, E.; Sevanian, A.; Poli, G. Lipid oxidation products in cell signaling. Free Radic. Biol. Med. 2000, 28, 1370–1378. [Google Scholar] [CrossRef]
- Poli, G.; Biasi, F.; Leonarduzzi, G. 4-hydroxynonenal-protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol. Asp. Med. 2008, 29, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, N.; Zarkovic, K.; Kralj, M.; Borovic, S.; Sabolovic, S.; Blazi, M.P.; Cipak, A.; Pavelic, K. Anticancer and antioxidative effects of micronized zeolite clinoptilolite. Anticancer Res. 2003, 23, 1589–1595. [Google Scholar] [PubMed]
- Pecorelli, A.; Ciccoli, L.; Signorini, C.; Leoncini, S.; Giardini, A.; D’Esposito, M.; Filosa, S.; Hayek, J.; De Felice, C.; Valacchi, G. Increased levels of 4hne-protein plasma adducts in rett syndrome. Clin. Biochem. 2011, 44, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Leoncini, S.; De Felice, C.; Signorini, C.; Cerrone, C.; Valacchi, G.; Ciccoli, L.; Hayek, J. Non-protein-bound iron and 4-hydroxynonenal protein adducts in classic autism. Brain Dev. 2013, 35, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Frantseva, M.V.; Perez Velazquez, J.L.; Tsoraklidis, G.; Mendonca, A.J.; Adamchik, Y.; Mills, L.R.; Carlen, P.L.; Burnham, M.W. Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience 2000, 97, 431–435. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (hne) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.J.; Lovell, M.A.; Markesbery, W.R.; Uchida, K.; Mattson, M.P. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J. Neurochem. 1997, 68, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Montine, K.S.; Reich, E.; Neely, M.D.; Sidell, K.R.; Olson, S.J.; Markesbery, W.R.; Montine, T.J. Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in alzheimer disease is associated with apoe genotype. J. Neuropathol. Exp. Neurol. 1998, 57, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Theard, M.A.; Baughman, V.L.; Wang, Q.; Pelligrino, D.A.; Albrecht, R.F. The role of nitric oxide in modulating brain activity and blood flow during seizure. Neuroreport 1995, 6, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Bosnak, M.; Ayyildiz, M.; Yildirim, M.; Agar, E. The role of nitric oxide in the anticonvulsant effects of pyridoxine on penicillin-induced epileptiform activity in rats. Epilepsy Res. 2007, 76, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sardo, P.; Ferraro, G. Modulatory effects of nitric oxide-active drugs on the anticonvulsant activity of lamotrigine in an experimental model of partial complex epilepsy in the rat. BMC Neurosci. 2007, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Osonoe, K.; Mori, N.; Suzuki, K.; Osonoe, M. Antiepileptic effects of inhibitors of nitric oxide synthase examined in pentylenetetrazol-induced seizures in rats. Brain Res. 1994, 663, 338–340. [Google Scholar] [CrossRef]
- Murashima, Y.L.; Yoshii, M.; Suzuki, J. Ictogenesis and epileptogenesis in el mice. Epilepsia 2002, 43 (Suppl. 5), 130–135. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Akaike, T.; Maeda, H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J. Biol. Chem. 2000, 275, 32467–32474. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.; Liang, L.P.; Rivard, C.; Patel, M. Temporal and spatial increase of reactive nitrogen species in the kainate model of temporal lobe epilepsy. Neurobiol. Dis. 2014, 64, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solowiej, E.; Sobaniec, W. The effect of antiepileptic drug therapy on antioxidant enzyme activity and serum lipid peroxidation in young patients with epilepsy. Neurol. Neurochir. Pol. 2003, 37, 991–1003. [Google Scholar] [PubMed]
- Peker, E.; Oktar, S.; Ari, M.; Kozan, R.; Dogan, M.; Cagan, E.; Sogut, S. Nitric oxide, lipid peroxidation, and antioxidant enzyme levels in epileptic children using valproic acid. Brain Res. 2009, 1297, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Martinc, B.; Grabnar, I.; Vovk, T. The role of reactive species in epileptogenesis and influence of antiepileptic drug therapy on oxidative stress. Curr. Neuropharmacol. 2012, 10, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Piskorska, B.; Czuczwar, S.J.; Borowicz, K.K. Nitric oxide, epileptic seizures, and action of antiepileptic drugs. CNS Neurol. Disord. Drug Targets 2011, 10, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Ramalingam, K.; Kumar, R.V. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure 2012, 21, 780–784. [Google Scholar] [CrossRef] [PubMed]




| No. | Age (Years) | Gender | Time Seizure Evolution (Years) | Seizure Frequency/Month | Seizure Localization | Drugs |
|---|---|---|---|---|---|---|
| 1 | 21 | M | 17 | 4,5 | ET | PNT, PM, CBZ |
| 2 | 19 | M | 16 | 16 | ET | LMG, CBZ, CLON |
| 3 | 48 | F | 35 | 13 | ET | CBZ, LMG |
| 4 | 19 | M | 17 | 2 | T | TP |
| 5 | 17 | M | 10 | 7 | ET | CBZ, LMG, CLB |
| 6 | 22 | M | 16 | 2 | T | CBZ |
| 7 | 57 | M | 11 | 12 | T | MV |
| 8 | 38 | F | 18 | 1 | ET | MV |
| 9 | 46 | F | 46 | 4 | ET | PBT, PNT, CBZ |
| 10 | 28 | F | 14 | 90 | ET | CBZ, CLON |
| 11 | 30 | M | 18 | 12 | T | CBZ, LMG |
| 12 | 38 | M | 30 | 3 | T | CBZ |
| 13 | 36 | M | 27 | 2 | T | CBZ, MV |
| 14 | 31 | F | 28 | 120 | ET | LMG, CLON |
| 15 | 27 | M | 10 | 1 | T | MV |
| 16 | 48 | M | 4 | 30 | T | LMG |
| 17 | 22 | F | 7 | 30 | ET | CBZ, LMG |
| 18 | 16 | M | 9 | 360 | T | TOP, LVT, CLON |
| Oxidative Stress Parameters | Age at Onset of Seizure | Frequency of Seizures per Month | Number of Drugs |
|---|---|---|---|
| MDA | −0.5500 * | 0.0484 | 0.5351 * |
| AOPP | −0.1050 | 0.1048 | −0.0291 |
| AGEs | −0.4741 | −0.3939 | 0.3028 |
| NO | −0.3416 | −0.3818 | 0.5843 * |
| Uric Acid | −0.1853 | −0.1996 | 0.0529 |
| SOD | 0.1503 | −0.2642 | −0.2594 |
| Glutathione | −0.1652 | −0.2892 | 0.2182 |
| Vitamin C | −0.2136 | −0.7110 * | 0.1912 |
| 3−NT | 0.3878 | 0.7565 * | −0.0670 |
| 4−HNE | −0.0852 | 0.3246 | 0.4022 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorigados Pedre, L.; Gallardo, J.M.; Morales Chacón, L.M.; Vega García, A.; Flores-Mendoza, M.; Neri-Gómez, T.; Estupiñán Díaz, B.; Cruz-Xenes, R.M.; Pavón Fuentes, N.; Orozco-Suárez, S. Oxidative Stress in Patients with Drug Resistant Partial Complex Seizure. Behav. Sci. 2018, 8, 59. https://doi.org/10.3390/bs8060059
Lorigados Pedre L, Gallardo JM, Morales Chacón LM, Vega García A, Flores-Mendoza M, Neri-Gómez T, Estupiñán Díaz B, Cruz-Xenes RM, Pavón Fuentes N, Orozco-Suárez S. Oxidative Stress in Patients with Drug Resistant Partial Complex Seizure. Behavioral Sciences. 2018; 8(6):59. https://doi.org/10.3390/bs8060059
Chicago/Turabian StyleLorigados Pedre, Lourdes, Juan M. Gallardo, Lilia M. Morales Chacón, Angélica Vega García, Monserrat Flores-Mendoza, Teresa Neri-Gómez, Bárbara Estupiñán Díaz, Rachel M. Cruz-Xenes, Nancy Pavón Fuentes, and Sandra Orozco-Suárez. 2018. "Oxidative Stress in Patients with Drug Resistant Partial Complex Seizure" Behavioral Sciences 8, no. 6: 59. https://doi.org/10.3390/bs8060059
APA StyleLorigados Pedre, L., Gallardo, J. M., Morales Chacón, L. M., Vega García, A., Flores-Mendoza, M., Neri-Gómez, T., Estupiñán Díaz, B., Cruz-Xenes, R. M., Pavón Fuentes, N., & Orozco-Suárez, S. (2018). Oxidative Stress in Patients with Drug Resistant Partial Complex Seizure. Behavioral Sciences, 8(6), 59. https://doi.org/10.3390/bs8060059