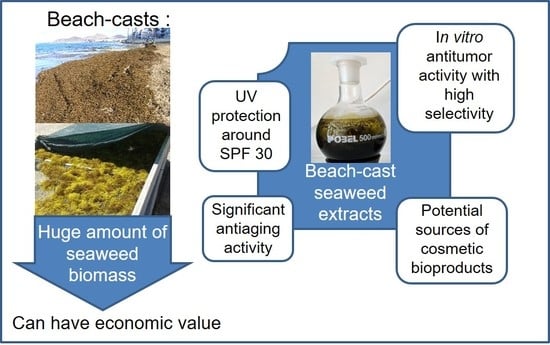
Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Harvesting, Treatment and Processing of Seaweed Beach-Casts
2.2. Algal Biomass Extraction with Organic Solvents
2.3. Preparation of Stock Solutions of Extracted Samples for Conducting Bioassays
2.4. Antibacterial Activity
2.5. Antifungal Activity
2.6. Anticancer Activity
2.7. Anticholinesterasic Activity
2.8. Antiaging Activity
2.8.1. DPPH Radical Scavenging Activity
2.8.2. ABTS Radical Scavenging Assay
2.8.3. Ferric Chelating Activity Assay
2.8.4. Inhibition of β-Carotene-Bleaching Assay
2.8.5. Determination of Sun Protection Factor (SPF)
2.8.6. Inhibition of Extracellular Matrix ECM and Skin-Degrading Enzymes
Hyaluronidase Inhibition Assay
Tyrosinase Inhibition Assay
Elastase Inhibition Assay
Collagenase Inhibition Assay
3. Results and Discussion
3.1. Antibacterial and Antifungal Activities
3.2. Anticancer Activity
3.3. Anticholinesterasic Activity
3.4. Antiaging Activity
3.4.1. Chelating and Antioxidant Activities
3.4.2. UV-Protection
3.4.3. Inhibition of ECM and Skin-Degrading Enzymes
Hyaluronidase Inhibition
Tyrosinase Inhibition
Elastase Inhibition
Collagenase Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2:2′ Azinobis-(3 ethylbenzothiazoline 6 sulfonic acid) |
| AChE | acetylcholinesterase |
| ATCI | acetylthiocholine |
| BuChE | butyrylcholinesterase |
| BuTCI | butyrythiocholine |
| DMAB | 4-dimethyaminobenzaldehyde |
| DMSO | dimethyl sulfoxide |
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl |
| DTNB | 5,5′-dithiobis-[2-nitrobenzoic acid] |
| ECM | Extracellular Matrix |
| FALGPA | N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala |
| MAAPVCK | N-Methoxysucinil-Ala-Ala-Pro-Val-chloromethylketone |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| PLE | pressurized liquid extraction |
| ROS | reactive oxygen species |
| SFE | supercritical fluid extraction |
| SI | selectivity index |
| SPF | sun protection factor |
| TES | N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid |
| TSB | tryptic soy broth |
| YPD | yeast extract peptone dextrose |
References
- Holden, J.J.; MacNeill, S.K.; Juanes, F.; Dudas, S.E. Beach-cast deposition and commercial harvesting of a non-indigenous alga, Mazzaella japonica: Implications for macrofauna communities in Baynes Sound, British Columbia. Estuar. Coast. Shelf. Sci. 2018, 210, 162–171. [Google Scholar] [CrossRef]
- Zemke-White, W.L.; Speed, S.R.; McClary, D.J. Beach cast seaweed: A review. N. Z. Fish. Assess. Rep. 2005, 44, 1175–1584. [Google Scholar]
- Andersen, J.H.; Carstensen, J.; Conley, D.J.; Dromph, K.; Fleming-Lehtinen, V.; Gustafsson, B.G.; Josefson, A.B.; Norkko, A.; Villnäs, A.; Murray, C. Long-term temporal and spatial trends in eutrophication status of the Baltic Sea. Biol. Rev. 2017, 92, 135–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, K.; Schreider, M.; McArthur, L.; Schreider, S. Changes in the water quality characteristics during a macroalgal bloom in a coastal lagoon. Ocean Coast. Manag. 2015, 118A, 32–36. [Google Scholar] [CrossRef]
- van Beukering, P.; Cesar, H.S.J. Ecological economic modeling of coral reefs: Evaluating tourist overuse at Hanauma Bay and algae blooms at the Kihei Coast, Hawaii. Pac. Sci. 2004, 58, 243–260. [Google Scholar] [CrossRef] [Green Version]
- Portillo, E. Relationship between type of wave exposure and seagrass losses (Cymodocea nodosa) in the south of Gran Canaria (Canary Islands). Oceanol. Hydrobiol. Stud. 2014, 43, 29–40. [Google Scholar] [CrossRef]
- Williams, A.; Micallef, A. Beach Management: Principles and Practices; Earthscan: London, UK, 2009. [Google Scholar] [CrossRef]
- Portillo-Hahnefeld, E. Arribazones de Algas y Plantas Marinas en Gran Canaria. Características, Gestión y Posibles Usos; Ed. Intituto Tecnologico de Canarias: Santa Lucía, Spain, 2008; ISBN 978-84-691-5105-I. [Google Scholar]
- Tanna, B.; Mishra, A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [Green Version]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C—Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Guerrero, A.J.; Rodriguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2014–2015: Marine compounds with antibacterial, antidiabetic, antifungal, anti-Inflammatory, antiprotozoal, antituberculosis, antiviral, and anthelmintic activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2020, 18, e5. [Google Scholar] [CrossRef] [Green Version]
- Milledge, J.J.; Harvey, P.J. Golden tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Masri, M.A.; Younes, S.; Haack, M.; Qoura, F.; Mehlmer, N.; Bruck, T. A Seagrass-based biorefinery for generation of single-cell oils for biofuel and oleochemical production. Energy Technol. 2018, 6, 1026–1038. [Google Scholar] [CrossRef] [Green Version]
- Portillo, E.; Suárez, A.; Mendoza, H. Procedimiento Para el Tratamiento de Algas y Fanerógamas Marinas. Patent ES2346839 A1, 2010. [Google Scholar]
- Nunes, N.; Valente, S.; Ferraz, B.M.C.; Pinheiro de Carvalho, M.A.A. Constructing ethanol-derived bioactive extracts using the brown seaweed Zonaria tournefortii (J.V.Lamouroux) Montagne performed with Timatic extractor by means of response surface methodology (RSM). J. Appl. Phycol. 2020, in press. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Koraichi-Ibnsouda, S. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharmac. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Geran, R.I.; Greenberg, N.H.; McDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumour and other biological systems (3rd edition). Cancer Chemother. Rep. 1972, 3, 1–103. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General cytotoxicity assessment by means of the MTT assay. Methods Mol. Biol. 2015, 1250, 333–348. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Arruda, M.; Viana, H.; Rainha, N.; Neng, N.R.; Rosa, J.S.; Nogueira, J.M.; Barreto, M.C. Anti-acetylcholinesterase and antioxidant activity of essential oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules 2012, 17, 3082–3092. [Google Scholar] [CrossRef] [Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Barreira, J.C.; Ferreira, I.C.; Oliveira, M.B.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Lu, Y.; Khoo, T.J.; Wiart, C. Antioxidant activity determination of citronellal and crude extracts of Cymbopogon citratus by 3 different methods. Pharmacol. Pharm. 2014, 5, 395. [Google Scholar] [CrossRef] [Green Version]
- Mansur, J.S.; Breder, M.N.R.; Mansur, M.C.A.; Azulay, R.D. Determination of sun protection factor by spectrophotometric methods. Brazil. Ann. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Santos, E.P.; Freitas, Z.M.; Souza, K.R.; Garcia, S.; Vergnanini, A. In vitro and in vivo determinations of sun protection factors of sunscreen lotions with octyl methoxycinnamate. Int. J. Cosmet. Sci. 1999, 21, 1–5. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. Comparison of in vivo and in vitro testing of sunscreening formulas. J. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.R.; Kanda, Y.; Tanaka, A.; Manabe, H.; Nohara, T.; Yokomizo, K. Anti-hyaluronidase activity in vitro and amelioration of mouse experimental dermatitis by tomato saponin, esculeoside A. J. Agric. Food Chem. 2016, 64, 403–408. [Google Scholar] [CrossRef]
- Shimizu, K.; Kondo, R.; Sakai, K.; Lee, S.H.; Sato, H. The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med. 1998, 64, 408–412. [Google Scholar] [CrossRef]
- Manosroi, A.; Jantrawut, P.; Akihisa, T.; Manosroi, W.; Manosroi, J. In vitro anti-aging activities of Terminalia chebula gall extract. Pharm. Biol. 2010, 48, 469–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [PubMed]
- Mandl, I.; MacLennan, J.D.; Howes, E.L.; DeBellis, R.H.; Sohler, A. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J. Clin. Investig. 1953, 32, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Heiman, F.L.W.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Yelin, I.; Kishony, R. Antibiotic resistance. Cell 2018, 172, 1136–1136. [Google Scholar] [CrossRef]
- Ritcher, M.F.; Hergenrother, P.J. The challenge of converting Gram-positive-only compounds into broad-spectrum antibiotics. Ann. N. Y. Acad. Sci. 2019, 35, 18–38. [Google Scholar] [CrossRef] [Green Version]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2017; Available online: https://seer.cancer.gov/csr/1975_2015/ (accessed on 15 April 2020).
- Ye, B.R.; Kim, J.; Kim, M.S.; Jang, J.; Oh, C.; Kang, D.H.; Qian, Z.J.; Jung, W.K.; Choi, I.W.; Heo, S.J. Induction of apoptosis by the tropical seaweed Pylaiella littoralis in HT-29 Cells via the mitochondrial and MAPK pathways. Ocean. Sci. J. 2013, 48, 339–348. [Google Scholar] [CrossRef]
- Hao, S.; Yan, Y.; Li, S.; Zhao, L.; Zhang, C.; Liu, L.; Wang, C. The in vitro anti-tumor activity of phycocyanin against non-small cell lung cancer cells. Mar. Drugs 2018, 16, 178. [Google Scholar] [CrossRef] [Green Version]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Moustafa, S.M.A.; Menshawi, B.M.; Wassel, G.M.; Mahmoud, K.; Mounier, M.M. Screening of some plants in Egypt for their cytotoxicity against four human cancer cell lines. Int. J. PharmaTech. Res. 2014, 3, 1074–1084. [Google Scholar]
- Taskin, E.; Caki, Z.; Osturk, M.; Taskinm, E. Assessment of in vitro antitumoral and antimicrobial activities of marine algae harvested from the eastern Mediterranean Sea. Afr. J. Biotechnol. 2010, 9, 4272–4277. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Khanavi, M.; Nabavi, M.; Sadati, N.; Ardekani, M.S.; Sohrabipour, J.; Nabavi, S.M.B.; Ghaelis, P.; Ostad, S.N. Cytotoxic activity of some marine brown algae against cancer cell lines. Biol. Res. 2010, 43, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Rocha, D.H.A.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef] [Green Version]
- Kubanek, J.; Paul, R.; Jensen, P.R.; Keifer, P.A.; Sullards, M.C.; Collins, D.O.; Fenical, W. Seaweed resistance to microbial attack: A targeted chemical defense against marine fungi. Proc. Natl. Acad. Sci. USA 2003, 12, 6916–6921. [Google Scholar] [CrossRef] [Green Version]
- Cavdar, H.; Senturk, M.; Guney, M.; Durdagi, S.; Kayik, G.; Supuran, C.T.; Ekinci, D. Inhibition of acetylcholinesterase and butyrylcholinesterase with uracil derivatives: Kinetic and computational studies. J. Enzyme. Inhib. Med. Chem. 2019, 34, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Kannan, R.R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; Staden, J.V. Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Aqueous–ethanol extracts of some South African seaweeds inhibit beta-amyloid aggregation, cholinesterases, and beta-secretase activities in vitro. J. Food Biochem. 2019, 43, e12870. [Google Scholar] [CrossRef]
- Rathnayake, A.U.; Abuine, R.; Kim, Y.-J.; Byun, H.-G. Anti-Alzheimer’s materials isolated from marine bio-resources: A review. Curr. Alzheimer. Res. 2019, 16, 895–906. [Google Scholar] [CrossRef]
- Lee, J.; Jun, M. Dual BACE1 and cholinesterase inhibitory effects of phlorotannins from Ecklonia cava—An in vitro and in silico study. Mar. Drugs 2019, 17, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of Alzheimer’s disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S-K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Imokawa, G.; Nakajima, H.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: Over-expression of neprilysin plays an essential role. Int. J. Mol. Sci. 2015, 16, 7776–7795. [Google Scholar] [CrossRef] [Green Version]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Badarinath, A.V.; Rao, K.M.; Chetty, C.M.S.; Ramkanth, S.; Rajan, T.V.S.; Gnanaprakash, K. A review of in vitro antioxidant methods: Comparisons, correlations and considerations. Int. J. PharmTech Res. 2010, 2, 1276–1285. [Google Scholar]
- Gomez-Zavaglia, A.; Lage, M.A.P.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef] [Green Version]
- Sanjeewa, K.K.A.; Kima, E.-A.; Son, K.-T.; Jeona, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B 2016, 162, 100–105. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Ko, J.Y.; Jeon, Y.-J.; Ryu, B. In vitro and in vivo antioxidant activities of polysaccharides isolated from celluclast-assisted extract of an edible brown seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Koba, K.; Hikihara, R.; Ishimaru, M.; Shibata, T.; Hatate, H.; Tanaka, R. Analysis of functional components and radical scavenging activity of 21 algae species collected from the Japanese coast. Food Chem. 2018, 255, 147–156. [Google Scholar] [CrossRef]
- Amano, S. Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp. Dermatol. 2016, 25, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Fayad, S.; Nehmé, R.; Tannoury, M.; Lesellier, E.; Pichon, C.; Morina, P. Macroalga Padina pavonica water extracts obtained by pressurized liquid extraction and microwave-assisted extraction inhibit hyaluronidase activity as shown by capillary electrophoresis. J. Chromatogr. A 2017, 1497, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Girish, K.S.; Kemparaju, K.; Nagaraju, S.; Vishwanath, B.S. Hyaluronidase inhibitors: A biological and therapeutic perspective. Curr. Med. Chem. 2009, 16, 2261–2288. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Nurrochmad, A.; Wirasti, W.; Dirman, A.; Lukitaningsih, E.; Rahmawati, A.; Fakhrudin, N. Effects of antioxidant, anti-collagenase, anti-elastase, anti-tyrosinase of the extract and fraction from Turbinaria decurrens Bory. Indones. J. Pharm. 2018, 29, 188–197. [Google Scholar] [CrossRef]
- Neeley, E.; Fritch, G.; Fuller, A.; Wolfe, J.; Wright, J.; Flurkey, W. Variations in IC50 values with purity of mushroom tyrosinase. Int. J. Mol. Sci. 2009, 10, 3811–3823. [Google Scholar] [CrossRef] [Green Version]
- Mahomoodally, M.F.; Bibi Sadeer, N.; Zengin, G.; Cziáky, Z.; Jekő, J.; Diuzheva, A.; Sinan, K.I.; Palaniveloo, K.; Kim, D.H.; Rengasamy, K.R.R. In vitro enzyme inhibitory properties, secondary metabolite profiles and multivariate analysis of five seaweeds. Mar. Drugs 2020, 18, 198. [Google Scholar] [CrossRef] [Green Version]
- Sari, D.M.; Anwar, E.; Nurjanah, A.E.A. Antioxidant and tyrosinase inhibitor activities of ethanol extracts of brown seaweed (Turbinaria conoides) as lightening ingredient. Pharmacogn. J. 2019, 11, 379–382. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.V.; Kim, S.-K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-X.; Wijesekaraa, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Ta, Q.V.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef]
- Mansauda, K.L.R.; Anwar, E.; Nurhayati, T. Antioxidant and anti-collagenase activity of Sargassum plagyophyllum extract as an anti-wrinkle cosmetic ingredient. Pharmacog. J. 2018, 10, 932–936. [Google Scholar] [CrossRef] [Green Version]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.B.W.; Kim, M.J.; Jeong, S.M.; Ahn, D.H. Free radical scavenging and anticollagenase properties of sargachromanol I from Myagropsis myagroides. Indian J. Pharm. Sci. 2019, 81, 561–565. [Google Scholar] [CrossRef] [Green Version]








| Wavelength (λ) | EE(λ) × I(λ) Value |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0837 |
| 320 | 0.0180 |
| IC50 (µg/mL) | |||||
|---|---|---|---|---|---|
| Sample | Lung NCIH1299 | Prostate DU145 | Breast T47D | Colon HT29 | Vero |
| 1.E | 33.77 | 36.80 | 19.28 | 74.29 | |
| 1.E.1 | 17.27 | 23.60 | 17.04 | 46.20 | 26.50 |
| 1.E.2 | 12.28 | - | 14.74 | 32.14 | 63.20 |
| 1.M | 24.60 | 28.50 | 16.04 | - | 80.00 |
| 1.M.1 | 12.50 | 25.40 | 21.90 | 24.40 | 59.30 |
| 1.M.2 | 27.60 | - | 33.60 | >80.00 | |
| 2.E | - | - | 22.85 | - | >80.00 |
| 2.M.1 | 23.90 | - | 39.60 | >80.00 | |
| 4.M | 26.43 | - | - | - | >80.00 |
| 5.E | 25.45 | - | - | - | >80.00 |
| 5.M.1 | 33.30 | - | 23.11 | >80.00 | |
| 6.M | 28.13 | - | - | - | >80.00 |
| 7.E | 31.51 | - | - | - | >80.00 |
| 10.E | 30.92 | - | 80.30 | - | >80.00 |
| 10.M | - | - | 77.25 | >80.00 | |
| 11.E | 13.13 | - | 38.60 | - | >80.00 |
| 12.E | 11.38 | - | 33.87 | - | >80.00 |
| 12.E.1 | 20.77 | 39.30 | 20.04 | 47.90 | 53.10 |
| Sample | Lung NCIH1299 | Prostate DU145 | Breast T47D | Colon HT29 |
|---|---|---|---|---|
| 1.E | 2.20 | 2.02 | 3.85 | |
| 1.E.1 | 1.53 | 1.12 | 1.55 | 0.57 |
| 1.E.2 | 5.14 | - | 4.28 | 1.97 |
| 1.M | 3.25 | 2.80 | 4.99 | - |
| 1.M.1 | 4.74 | 2.33 | 2.71 | 2.43 |
| 1.M.2 | 2.89 | - | 2.38 | |
| 2.E | - | - | 3.50 | - |
| 2.M.1 | 3.34 | - | 2.02 | |
| 4.M | 3.02 | - | - | - |
| 5.E | 3.14 | - | - | - |
| 5.M.1 | 2.40 | - | 3.46 | |
| 6.M | 2.84 | - | - | - |
| 7.E | 2.53 | - | - | - |
| 10.E | 2.58 | - | 0.99 | - |
| 10.M | - | - | 1.03 | |
| 11.E | 6.09 | - | 2.07 | - |
| 12.E | 7.03 | - | 2.36 | - |
| 12.E.1 | 2.55 | 1.35 | 2.65 | 1.11 |
| Sample | SPF | Sample | SPF |
|---|---|---|---|
| 3.E | 32.95 ± 0.49 | 8.M | 32.88 ± 1.30 |
| 3.M | 33.93 ± 1.83 | 9.E | 33.37 ± 1.25 |
| 4.E | 31.27 ± 0.52 | 9.M | 33.54 ± 2.23 |
| 4.M | 33.15 ± 1.95 | 10.E | 31.34 ± 0.48 |
| 5.E | 34.03 ± 1.03 | 10.M | 30.48 ± 0.59 |
| 5.M | 33.38 ± 1.68 | 11.E | 33.19 ± 0.52 |
| 6.E | 32.41 ± 1.34 | 11.M | 34.37 ± 3.32 |
| 6.M | 34.37 ± 0.95 | 12.E | 31.31 ± 0.88 |
| 7.E | 31.12 ± 0.78 | 12.M | 32.48 ± 1.51 |
| 7.M | 32.82 ± 0.34 | Standard | 32.86 ± 1.46 |
| 8.E | 33.62 ± 1.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zárate, R.; Portillo, E.; Teixidó, S.; Carvalho, M.A.A.P.d.; Nunes, N.; Ferraz, S.; Seca, A.M.L.; Rosa, G.P.; Barreto, M.C. Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia. Appl. Sci. 2020, 10, 5831. https://doi.org/10.3390/app10175831
Zárate R, Portillo E, Teixidó S, Carvalho MAAPd, Nunes N, Ferraz S, Seca AML, Rosa GP, Barreto MC. Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia. Applied Sciences. 2020; 10(17):5831. https://doi.org/10.3390/app10175831
Chicago/Turabian StyleZárate, Rafael, Eduardo Portillo, Sílvia Teixidó, Miguel A. A. Pinheiro de Carvalho, Nuno Nunes, Sónia Ferraz, Ana M. L. Seca, Gonçalo P. Rosa, and Maria Carmo Barreto. 2020. "Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia" Applied Sciences 10, no. 17: 5831. https://doi.org/10.3390/app10175831
APA StyleZárate, R., Portillo, E., Teixidó, S., Carvalho, M. A. A. P. d., Nunes, N., Ferraz, S., Seca, A. M. L., Rosa, G. P., & Barreto, M. C. (2020). Pharmacological and Cosmeceutical Potential of Seaweed Beach-Casts of Macaronesia. Applied Sciences, 10(17), 5831. https://doi.org/10.3390/app10175831