Recent Advances in Optical Detection of Aminoglycosides
Abstract
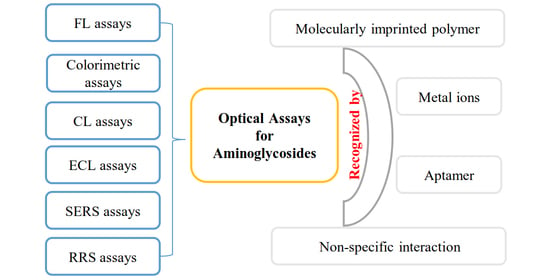
:1. Introduction
1.1. The History of Aminoglycosides
1.2. The Danger of Aminoglycosides
1.3. Conventional Detection Methods
1.4. Recent Advances in Optical Detection of Aminoglycosides
2. Fluorescent Assays
2.1. Turn-Off Fluorescent Analysis Strategies
2.2. Turn-On Fluorescent Analysis Strategies
2.3. Ratio Fluorescent Analysis Strategies
2.4. Discussion
3. Colorimetric Assays
3.1. Colorimetric Reactions Used in AG Detection
3.2. Based on In Situ Generation of Nanoparticles
3.3. Colorimetric Biosensor Strips
3.4. Based on Target-Induced Aggregation of Metal Nanoparticles
3.5. Discussion
4. Other Optical Assays
4.1. Chemiluminescence Assays
4.2. Electrochemiluminescence
4.3. Surface-Enhanced Raman Spectroscopy
4.4. Resonance Rayleigh Scattering
4.5. Discussion
5. Amplification Strategies
5.1. Nanomaterials as the Signal Amplification Carrier
5.2. Exonuclease III-Assisted Target Recycling
5.3. Hybridization Chain Reaction
5.4. Rolling Circle Amplification
5.5. Double Amplification Strategies
5.6. Discussion
6. Multiplex Detection of Antibiotics
6.1. Based on Multiplex Labels
6.2. Based on Spatial Resolution
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) |
| AGs | Aminoglycosides |
| Ag NCs | Silver nanoclusters |
| ALP | Alkaline phosphatase |
| Ag NPs | Silver nanoparticles |
| APTES | Aminopropyltriethoxysilane |
| Au NCs | Gold nanoclusters |
| Au NPs | Gold nanoparticles |
| BSA | Bovine serum albumin |
| CDs | Carbon dots |
| CE | Capillary electrophoresis |
| CL | Chemiluminescent |
| CNTs | Carbon nanotubes |
| dsDNA | Double-strand DNA |
| dNTP | Deoxy-ribonucleoside triphosphate |
| ECL | Electrogenerated chemiluminescence |
| EGCG | Epigallocatechin gallate |
| ELISA | Enzyme-linked immunosorbent assay |
| ELSD | Evaporative light-scattering detection |
| Exo III | Exonuclease III |
| FAM | Carboxyfluorescein |
| FL | Fluorescence |
| FRET | Fluorescence resonance energy transfer |
| GC | Gas chromatography |
| GFP | Fluorescent protein |
| GO | Graphene oxide |
| GQDs | Graphene oxide quantum dots |
| HCA | Hierarchical clustering analysis |
| HCR | Hybridization chain reaction |
| HPLC | High-performance liquid chromatography |
| HRP | Horseradish peroxidase |
| LOD | Limit of detection |
| LFA | Lateral flow assay |
| MIPs | Molecular imprinting polymers |
| MOFs | Metal-organic frameworks |
| MRL | Maximum residue limit |
| MS | Mass spectrometry |
| OPD | o-phenylenediamine |
| PAD | Pulsed amperometric detectors |
| PATP | 4-aminothiophenol |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| PDDA | Poly(diallyl dimethyl ammonium chloride) |
| POCT | Point of care test |
| QDs | Quantum dots |
| RCA | Rolling circle amplification |
| RRS | Resonance Rayleigh scattering |
| RRS-ET | Resonance Rayleigh scattering-energy transfer |
| SDS | Sodium dodecyl sulfate |
| SELEX | Systemic evolution of ligands by exponential enrichment |
| SERS | Surface-enhanced Raman spectroscopy |
| SPR | Surface plasmon resonance |
| SSB | Single-stranded DNA-binding protein |
| ssDNA | Single-strand DNA |
| TAMRA | Carboxytetramethylrhodamine |
| TCPP | Tetrakis(4-carboxyphenyl)porphyrin |
| TEOS | Tetraethoxysilane |
| TMB | Tetramethyl benzidine |
Appendix A
| Target | Recognition Element | Detection Mode/Principle | Linear Range (nM) | LOD (nM) | Time (min) | ** Selectivity | Real Sample (Recovery, %) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||
| Kanamycin | Aptamer | OFF/based on DNA-binding Dye | 0.6–20.0 | 0.33 | 65 | ◯ | ◯ | - | Milk (-) | [24] |
| OFF/based on DNA-binding Dye | 1–300,000 | 0.3 | 20 | - | ◯ | ◯ | Milk (94–105) | [25] | ||
| OFF/evanescent wave aptasensor | 200–200,000 | 26 | 10 | × | ◯ | - | Milk (91–97) | [27] | ||
| MIP and aptamer | OFF/target-induced FL quenching | 85.8–17,160 | 1.1 | 15–25 | ◯ | ◯ | ◯ | Milk (88–116) | [29] | |
| Aptamer | ON/FERT between CDs and MoS2 | 4000–25,000 | 1100 | - | ◯ | ◯ | ◯ | Milk (85–102) | [40] | |
| ON/FERT between Ag NCs and Au NPs | 5–50 | 1.0 | 30 | - | ◯ | - | Milk (90–95) | [17] | ||
| ON/FERT between FAM and Au NPs | 0.8–350 | 0.3 | 140 | ◯ | ◯ | - | Milk (99–105) | [41] | ||
| ON/FERT between FAM and CNTs | 1–50 | 0.4 | 75 | ◯ | ◯ | - | Milk (98–103) | [42] | ||
| ON/FERT between FAM and graphene | 0.001–0.020 | 0.001 | 55 | ◯ | ◯ | - | Milk (99–103) | [44] | ||
| ON/FRET between FAM and Dabcyl | 100–600 | 13.52 | 32 | ◯ | ◯ | ◯ | Milk (101–109) | [36] | ||
| ON/FRET between GQDs and BHQ1 | 0.017–154.4 | 0.01 | 95 | ◯ | ◯ | ◯ | Milk (97–103) | [37] | ||
| ON/in situ generation of fluorophore | 75–8000 | 36 | 80 | × | ◯ | - | Milk and honey (92–103) | [35] | ||
| Aptamer | RATIO/FRET between Cy3 and Cy5 | 0.18–5 | 0.18 | - | ◯ | ◯ | ◯ | Milk (-) | [38] | |
| Streptomycin | Aptamer | OFF/based on DNA-binding dye | 100–100,000 | 94 | 24 | ◯ | ◯ | ◯ | Milk and chicken (94–110) | [26] |
| Neomycin | MIPs | OFF/target-induced FL quenching | 3.3–1627 | 0.26 | 60 | ◯ | - | ◯ | Milk (77–94) | [30] |
| Lincomycin | MIPs | OFF/target induced FL quenching | 22.5–225 | 16.2 | 120 | - | ◯ | ◯ | Egg (96–99) | [32] |
| Target | Recognition Element | Detection Mechanism (Quantitative Parameters) | Linear Range (nM) | LOD (nM) | Time (min) | Selectivity ** | Sample (Recovery, %) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||
| Kanamycin | Aptamer | Lateral flow strip biosensor based on Au NPs (Optical density) | 1–30 | 0.0778 | 10 | ◯ | ◯ | - | Honey, milk and milk powder (-) | [51] |
| Aptamer | Salt-induced aggregation of Ag NPs (A404-A600) | 85–1000 | 4.5 | 20 | ◯ | ◯ | - | Milk (100–102) | [64] | |
| ECGG | Target-induced aggregation of Ag NPs (ΔA415) | 960–20,000 | 960 | 1 | - | ◯ | - | Milk (-) | [59] | |
| Chlortetracycline | Target-induced aggregation of Ag NPs (A540/400) | 0.12–0.48 | 0.12 | 20 | - | - | - | Water (-) | [61] | |
| Chitosan | Target-induced aggregation of Au NPs (A650/520) | 10–40,000 | 8 | 10 | - | ◯ | ◯ | Milk (100–101) | [65] | |
| AHMT | Target-induced aggregation of Au NPs (A650/520) | 5–100,100–2000 | 4 | 10 | - | ◯ | ◯ | Water and milk (98–120) | [66] | |
| Tobramycin | Aptamer | Salt-induced aggregation of Au NPs (A620/520) | 40–200 | 23.3 | 35 | ◯ | ◯ | ◯ | Milk and egg (93–105) | [56] |
| Aptamer | Salt-induced aggregation of Au NPs (ΔA520) | 100–1400 | 37.9 | 110 | - | - | - | Honey (98–99) | [57] | |
| Streptomycin | Gallic acid | Target-induced aggregation of Ag NPs (A560/A400) | 0.05–0.65 | 0.036 | 20 | × | ◯ | - | Milk (-) | [60] |
| Chlortetracycline | Target-induced aggregation of Ag NPs (A540/A400) | 2–11 | 2 | 20 | - | - | - | Water (-) | [61] | |
| Gentamicin | Cysteamine | Target-induced aggregation of Au NPs (A680/A520) | 12.45–200 | 12.45 | 15 | - | - | ◯ | Milk (-) | [58] |
| Target | Recognition Element | Detection Mechanism | Linear Range (nM) | LOD (nM) | Time (min) | Selectivity ** | Sample (Recovery, %) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||
| Kanamycin | Aptamer | Au NCs-catalyzed luminol CL reaction | 0.2–4.4 | 0.035 | 150 | ◯ | ◯ | ◯ | Milk (99–105) | [70] |
| Aptamer | Ag NPs-enhanced ECL reaction | 0.8–170 | 0.1 | 60 | ◯ | ◯ | - | Milk (96–107) | [73] | |
| Aptamer | SERS | 0.017–170 | 0.0015 | 40 | ◯ | ◯ | - | Milk (90–114) | [79] | |
| Aptamer | Opto-microfluidic system combined with SERS | 1–100 | 0.75 | 80 | ◯ | ◯ | - | Milk, orange juice, water (97–103) | [80] | |
| Streptomycin | Aptamer | DNAzyme-catalyzed luminol CL reaction | 0.0014–2.8 | 0.000092 | - | - | ◯ | ◯ | Cucumber and milk (97–103) | [71] |
| - | Strep–luminol–NaIO4–Mn2+ CL reaction | 10–1700 | 5.16 | - | - | - | ◯ | Milk (87–122) | [68] | |
| Lincomycin | Aptamer and MIPs | ECL-RET between CDs and Au-GO | 0.005–1 | 0.00016 | 10 | ◯ | ◯ | ◯ | Meat (90–105) | [74] |
| Neomycin | Antibody | SERS based on LFA | 1.4–140 | 0.0003 | 15 | ◯ | ◯ | ◯ | Milk (90–106) | [78] |
| Tobramycin | Aptamer | RRS-based on Cu2O | 0.50–17 | 0.19 | 26 | ◯ | ◯ | ◯ | Milk (98–103) | [82] |
| Target | Principle /Mode | Amplification Method | Linear Range (nM) | LOD (nM) | Time (min) | Selectivity ** | Sample (Recovery, %) | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||
| Kanamycin | HRP-catalyzed TMB reaction | Au NPs as amplification carrier | 1.7 × 10−6–1.7 × 105 | 1.7 × 10−6 | 150 | - | ◯ | ◯ | Milk (100–104) | [84] |
| Microfluidic chip combined with label-free FL | RCA | 1.36–17,000 | 0.51 | 160 | ◯ | ◯ | ◯ | Milk and fish (90–106) | [88] | |
| Turn-on FL | Target-assisted recycling and HCR | 2–5000 | 1.2 | 385 | ◯ | ◯ | - | Milk (-) | [86] | |
| DNAzyme-catalyzed colorimetric reaction | Exo III-assisted target recycling | 0.17–17,000 | 0.077 | 125 | ◯ | ◯ | - | Milk (91–109) | [20] | |
| Spilt G- Quadruplex DNAzyme catalyzed ABTS colorimetric reaction | Dual nicking enzyme signal amplification | 50–500,000 | 14.7 | 360 | ◯ | ◯ | ◯ | Milk (93–105) | [21] | |
| Label-free FL | Target recycling and HCR | 1.7–170,000 | 0.77 | 65 | ◯ | ◯ | ◯ | Milk, fish, and pork (90–114) | [22] | |
| Streptomycin | Colorimetric immunoassay based on [Fe(II)-Phen] system | Liposome | 1.7–34,000 | 0.68 | 130 | ◯ | ◯ | - | Milk, honey, muscle and kidney (86–114) | [85] |
References
- Nhung, N.T.; Cuong, N.V.; Thwaites, G.; Carrique-Mas, J. Antimicrobial usage and antimicrobial resistance in animal production in Southeast Asia: A review. Antibiotics 2016, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, E.S.; Kostrub, C.F.; Cass, R.T.; Moser, H.E.; Serio, A.W.; Miller, G.H. Aminoglycosides. In Antibiotic Discovery and Development; Dougherty, T.J., Pucci, M.J., Eds.; Springer Science+Business Media: New York, NY, USA, 2012; Volume 1, pp. 229–270. [Google Scholar]
- Huth, M.E.; Han, K.H.; Sotoudeh, K.; Hsieh, Y.J.; Effertz, T.; Vu, A.A.; Verhoeven, S.; Hsieh, M.H.; Greenhouse, R.; Cheng, A.G.; et al. Designer aminoglycosides prevent cochlear hair cell loss and hearing loss. J. Clin. Investig. 2015, 125, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Farouk, F.; Azzazy, H.M.E.; Niessen, W.M.A. Challenges in the determination of aminoglycoside antibiotics, a review. Anal. Chim. Acta 2015, 890, 21–43. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef] [Green Version]
- FuJii, Y.; Kaga, T.; Nishimura, K. Simultaneous determination of aminoglycoside residues in livestock and fishery products by phenylboronic acid solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Sci. 2019, 9, 961–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stead, D.A. Current methodologies for the analysis of aminoglycosides. J. Chromatogr. B 2000, 747, 69–93. [Google Scholar] [CrossRef]
- Jariwala, F.B.; Hibbs, J.A.; Zhuk, I.; Sukhishvili, S.A.; Attygalle, A.B. Rapid determination of aminoglycosides in pharmaceutical preparations by electrospray ionization mass spectrometry. J. Anal. Sci. Technol. 2020, 11, 2. [Google Scholar] [CrossRef]
- Nagatoishi, S.; Nojima, T.; Galezowska, E.; Gluszynska, A.; Juskowiak, B.; Takenaka, S. Fluorescence energy transfer probes based on the guanine quadruplex formation for the fluorometric detection of potassium ion. Anal. Chim. Acta 2007, 581, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.P.; Cao, Z.J.; Li, H.; Kai, M.; Lu, J.Z. Chemiluminescence platforms in immunoassay and DNA analyses. Anal. Sci. 2009, 25, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-based biosensors for antibiotic detection: A review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalev, M.; Kandasamy, J.; Skalka, N.; Belakhov, V.; Rosin-Arbesfeld, R.; Baasov, T. Development of generic immunoassay for the detection of a series of aminoglycosides with 6‘-OH group for the treatment of genetic diseases in biological samples. J. Pharm. Biomed. Anal. 2012, 75, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, S.L.; Zhang, F.F.; Luo, X.; Yang, B.C.; Jin, G.W.; Yan, J.Y.; Liang, X.M. Synthesis of molecularly imprinted polymer sorbents and application for the determination of aminoglycosides antibiotics in honey. J. Chromatogr. A 2013, 1313, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Uzun, L.; Ozaltin, N.; Denizli, A. Development of molecular imprinted nanosensor for determination of tobramycin in pharmaceuticals and foods. Talanta 2013, 120, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Fu, Y.; Lu, Z.; Sang, Z.; Liu, K.; Du, C.; Ma, L. Sub-10-nm multicolored gold nanoparticles for colorimetric determination of antibiotics via formation of interlocking rings. Microchim. Acta 2019, 186, 318. [Google Scholar] [CrossRef]
- Ye, T.; Peng, Y.; Yuan, M.; Cao, H.; Yu, J.; Li, Y.; Xu, F.A. “turn-on” fluorometric assay for kanamycin detection by using silver nanoclusters and surface plasmon enhanced energy transfer. Microchim. Acta 2019, 186, 40. [Google Scholar] [CrossRef]
- Lin, X.; Su, J.; Lin, H.; Sun, X.; Liu, B.; Kankala, R.K.; Zhou, S.-F. Luminescent carbon nanodots based aptasensors for rapid detection of kanamycin residue. Talanta 2019, 202, 452–459. [Google Scholar] [CrossRef]
- Liu, X.; Na, W.; Liu, Q.; Su, X. A novel label-free fluorescent sensor for highly sensitive detection of bleomycin based on nitrogen-doped graphene quantum dots. Anal. Chim. Acta 2018, 1028, 45–49. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, F.; Yu, A.; Lai, G. Aptamer biorecognition-triggered dnazyme liberation and exo III-assisted target recycling for ultrasensitive homogeneous colorimetric bioassay of kanamycin antibiotic. Chem. Commun. 2019, 55, 3959–3962. [Google Scholar] [CrossRef]
- Cui, X.; Li, R.; Liu, X.; Wang, J.; Leng, X.; Song, X.; Pei, Q.; Wang, Y.; Liu, S.; Huang, J. Low-background and visual detection of antibiotic based on target-activated colorimetric split peroxidase dnazyme coupled with dual nicking enzyme signal amplification. Anal. Chim. Acta 2018, 997, 1–8. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, J.; Wu, Y.; Hu, F.; Li, T.; Wang, Y.; Gan, N. A fluorometric aptamer method for kanamycin by applying a dual amplification strategy and using double y-shaped DNA probes on a gold bar and onmagnetite nanoparticles. Microchim. Acta 2019, 186, 120. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.M.; Noureldin, H.A.M.; Badr, I.H.A.; Saad, A.H.K. Simple spectrofluorimetric methods for determination of veterinary antibiotic drug (apramycin sulfate) in pharmaceutical preparations and milk samples. Spectroc. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, W.; Tan, S.; Chen, T. Label-free and simple g-quadruplex-based turn-off fluorescence assay for the detection of kanamycin. Anal. Lett. 2018, 51, 1718–1729. [Google Scholar] [CrossRef]
- Ma, L.; Sun, N.; Tu, C.H.; Zhang, Q.; Diao, A.P. Design of an aptamer-based fluorescence displacement biosensor for selective and sensitive detection of kanamycin in aqueous samples. RSC Adv. 2017, 7, 38512–38518. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Yu, Y.; Cao, Y.; Guo, M.; Zhu, D.; Dai, J.; Zheng, M. Point-of-care testing for streptomycin based on aptamer recognizing and digital image colorimetry by smartphone. Biosens. Bioelectron. 2018, 100, 482–489. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, C.; Wang, C.; Song, B.; Zhou, X.; Lou, X.; He, M. Evanescent wave aptasensor for continuous and online aminoglycoside antibiotics detection based on target binding facilitated fluorescence quenching. Biosens. Bioelectron. 2018, 102, 646–651. [Google Scholar] [CrossRef]
- Song, K.-M.; Cho, M.; Jo, H.; Min, K.; Jeon, S.H.; Kim, T.; Han, M.S.; Ku, J.K.; Ban, C. Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal. Biochem. 2011, 415, 175–181. [Google Scholar] [CrossRef]
- Geng, Y.; Guo, M.; Tan, J.; Huang, S.; Tang, Y.; Tan, L.; Liang, Y. A fluorescent molecularly imprinted polymer using aptamer as a functional monomer for sensing of kanamycin. Sens. Actuator B Chem. 2018, 268, 47–54. [Google Scholar] [CrossRef]
- Wan, Y.-c.; Liu, Y.-j.; Liu, C.; Ma, H.-t.; Yu, H.-f.; Kang, J.-w.; Gao, C.-l.; Wu, Z.-q.; Zheng, D.; Lu, B. Rapid determination of neomycin in biological samples using fluorescent sensor based on quantum dots with doubly selective binding sites. J. Pharm. Biomed. Anal. 2018, 154, 75–84. [Google Scholar] [CrossRef]
- Hassanzadeh, J.; Moghadam, B.R.; Sobhani-Nasab, A.; Ahmadi, F.; Rahimi-Nasrabadi, M. Specific fluorometric assay for direct determination of amikacin by molecularly imprinting polymer on high fluorescent g-C3N4 quantum dots. Spectroc. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 451–458. [Google Scholar] [CrossRef]
- Wu, P.; Du, Q.; Sun, Y.; Li, Z.; He, H. Mip-coated Eu(BTC) for the fluorometric determination of lincomycin in eggs. Anal. Methods 2019, 11, 4501–4510. [Google Scholar] [CrossRef]
- Karacaglar, N.N.Y.; Topcu, A.; Dudak, F.C.; Boyaci, I.H. Development of a green fluorescence protein (GFP)-based bioassay for detection of antibiotics and its application in milk. J. Food Sci. 2020, 85, 500–509. [Google Scholar] [CrossRef]
- Dehghani, S.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Lavaee, P.; Nejabat, M.; Abnous, K.; Taghdisi, S.M. A label-free fluorescent aptasensor for detection of kanamycin based on dsDNA-capped mesoporous silica nanoparticles and Rhodamine, B. Anal. Chim. Acta 2018, 1030, 142–147. [Google Scholar] [CrossRef]
- Belal, A.S.F.; Ismail, A.; Elnaggar, M.M.; Belal, T.S. Click chemistry inspired copper sulphide nanoparticle-based fluorescence assay of kanamycin using DNA aptamer. Spectroc. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 48–54. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, S.; Sun, H.; Su, R.; Sun, C.; Zhang, M. Development of structure-switching aptamers for kanamycin detection based on fluorescence resonance energy transfer. Front. Chem. 2019, 7, 29. [Google Scholar] [CrossRef]
- He, Y.; Wen, X.; Zhang, B.; Fan, Z. Novel aptasensor for the ultrasensitive detection of kanamycin based on graphene oxide quantum-dot-linked single-stranded DNA-binding protein. Sens. Actuator B Chem. 2018, 265, 20–26. [Google Scholar] [CrossRef]
- Pinto, I.A.; Toloza, C.A.T.; Almeida, J.M.S.; da Silva, A.R.; Larrude, D.G.; Aucelio, R.Q. Quantification of neomycin in rubella vaccine by off/on metal ion mediated photoluminescence from functionalized graphene quantum dots. Spectroc. Acta Part A Mol. Biomol. Spectrosc. 2019, 220, 117139. [Google Scholar] [CrossRef]
- Umrao, S.; Anusha, S.; Jain, V.; Chakraborty, B.; Roy, R. Smartphone-based kanamycin sensing with ratiometric FRET. RSC Adv. 2019, 9, 6143–6151. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.S.; Ma, T.C.; Ma, S.Y.; Liu, Y.J.; Tian, Y.P.; Wang, R.N.; Jiang, Y.B.; Hou, D.J.; Wang, J.L. Fluorometric determination of the antibiotic kanamycin by aptamer-induced FRET quenching and recovery between MoS2 nanosheets and carbon dots. Microchim. Acta 2017, 184, 203–210. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.H.; Ge, J.; Yang, R.; Zhang, L.; Qu, L.B.; Wang, H.Q.; Zhang, L. An aptamer-based signal-on bio-assay for sensitive and selective detection of Kanamycin A by using gold nanoparticles. Talanta 2015, 139, 226–232. [Google Scholar] [CrossRef]
- Liao, Q.G.; Wei, B.H.; Luo, L.G. Aptamer based fluorometric determination of kanamycin using double-stranded DNA and carbon nanotubes. Microchim. Acta 2017, 184, 627–632. [Google Scholar] [CrossRef]
- Su, D.; Wang, M.; Liu, Q.; Chen, J.; Su, X. Dual-emission ratio fluorescence detection of bleomycin based on nitrogen doped graphene quantum dots@gold nanoclusters assembly. Sens. Actuator B Chem. 2019, 290, 163–169. [Google Scholar] [CrossRef]
- Ha, N.-R.; Jung, I.-P.; La, I.-J.; Jung, H.-S.; Yoon, M.-Y. Ultra-sensitive detection of kanamycin for food safety using a reduced graphene oxide-based fluorescent aptasensor. Sci. Rep. 2017, 7, 40305. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Wen, F.; Guo, X.; Zheng, N.; Zhang, Y.; Li, S.; Zhao, S.; Liu, H.; Meng, L.; Xu, Q.; et al. Evaluation of an ELISA-based visualization microarray chip technique for the detection of veterinary antibiotics in milk. Food Control. 2019, 106, 106713. [Google Scholar] [CrossRef]
- Gong, L.; Zhao, Z.L.; Lv, Y.F.; Huan, S.Y.; Fu, T.; Zhang, X.B.; Shen, G.L.; Yu, R.Q. DNAzyme-based biosensors and nanodevices. Chem. Commun. 2015, 51, 979–995. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Wang, Q.; Tan, R. Kanamycin detection based on the catalytic ability enhancement of gold nanoparticles. Biosens. Bioelectron. 2017, 91, 262–267. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, B.; Dang, P.; Li, L.; Kong, J.; Zhang, X. Facile colorimetric assay of alkaline phosphatase activity using Fe(II)-phenanthroline reporter. Anal. Chim. Acta 2017, 950, 170–177. [Google Scholar] [CrossRef]
- Ji, Y.W.; Ren, M.L.; Li, Y.P.; Huang, Z.B.; Shu, M.; Yang, H.W.; Xiong, Y.H.; Xu, Y. Detection of aflatoxin B-1 with immunochromatographic test strips: Enhanced signal sensitivity using gold nanoflowers. Talanta 2015, 142, 206–212. [Google Scholar] [CrossRef]
- Leng, Y.; Hu, F.; Ma, C.; Du, C.; Ma, L.; Xu, J.; Lin, Q.; Sang, Z.; Lu, Z. Simple, rapid, sensitive, selective and label-free lincomycin detection by using HAucl4 and NaOH. RSC Adv. 2019, 9, 28248–28252. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zeng, J.; Tian, Y.; Zhou, N. An aptamer and functionalized nanoparticle-based strip biosensor for on-site detection of kanamycin in food samples. Analyst 2018, 143, 182–189. [Google Scholar] [CrossRef]
- Abedalwafa, M.A.; Tang, Z.; Qiao, Y.; Mei, Q.; Yang, G.; Li, Y.; Wang, L. An aptasensor strip-based colorimetric determination method for kanamycin using cellulose acetate nanofibers decorated DNA-gold nanoparticle bioconjugates. Microchim. Acta 2020, 187, 360. [Google Scholar] [CrossRef]
- Shayesteh, O.H.; Khosroshahi, A.G. A polya aptamer-based label-free colorimetric biosensor for the detection of kanamycin in human serum. Anal. Methods 2020, 12, 1858–1867. [Google Scholar] [CrossRef]
- Li, L.; Gu, Y.; Chen, Q.; Wu, P.; Li, N.; Chen, R.; He, H. Label-free silver nanoparticles for the determination of gentamicin. J. Nanosci. Nanotechnol. 2018, 18, 4501–4506. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, R.; Li, X. Colorimetric sensing for the determination of kanamycin based on gold nanoparticles. J. Anal. Sci. 2018, 34, 372–376. [Google Scholar]
- Ma, Q.; Wang, Y.; Jia, J.; Xiang, Y. Colorimetric aptasensors for determination of tobramycin in milk and chicken eggs based on DNA and gold nanoparticles. Food Chem. 2018, 249, 98–103. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Nie, J.; Zhao, S.; Tian, Y.; Zhou, N. Gold nanoparticle based photometric determination of tobramycin by using new specific DNA aptamers. Microchim. Acta 2018, 185, 4. [Google Scholar] [CrossRef]
- Gukowsky, J.C.; Tan, C.; Han, Z.; He, L. Cysteamine-modified gold nanoparticles as a colorimetric sensor for the rapid detection of gentamicin. J. Food Sci. 2018, 83, 1631–1638. [Google Scholar] [CrossRef]
- Singh, R.K.; Panigrahi, B.; Mishra, S.; Das, B.; Jayabalan, R.; Parhi, P.K.; Mandal, D. pH triggered green synthesized silver nanoparticles toward selective colorimetric detection of kanamycin and hazardous sulfide ions. J. Mol. Liq. 2018, 269, 269–277. [Google Scholar] [CrossRef]
- Ghodake, G.; Shinde, S.; Saratale, R.G.; Kadam, A.; Saratale, G.D.; Syed, A.; Marraiki, N.; Elgorban, A.M.; Kim, D.-Y. Silver nanoparticle probe for colorimetric detection of aminoglycoside antibiotics: Picomolar-level sensitivity toward streptomycin in water, serum, and milk samples. J. Sci. Food Agric. 2019, 100, 874. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Ghodake, G.; Shinde, S.; Kim, D.-Y.; Alyousef, A.A.; Arshad, M.; Syed, A.; Pant, D.; Shin, H.-S. Chlortetracycline-functionalized silver nanoparticles as a colorimetric probe for aminoglycosides: Ultrasensitive determination of kanamycin and streptomycin. Nanomaterials 2020, 10, 997. [Google Scholar] [CrossRef]
- ul Ain, N.; Aslam, Z.; Yousuf, M.; Waseem, W.A.; Bano, S.; Anis, I.; Ahmed, F.; Faizi, S.; Malik, M.I.; Shah, M.R. Green synthesis of methyl gallate conjugated silver nanoparticles: A colorimetric probe for gentamicin. New J. Chem. 2019, 43, 1972–1979. [Google Scholar] [CrossRef]
- Rezaei, H.; Rahimpour, E.; Khoubnasabjafari, M.; Jouyban-Gharamaleki, V.; Jouyban, A. A colorimetric nanoprobe based on dynamic aggregation of SDS-capped silver nanoparticles for tobramycin determination in exhaled breath condensate. Microchim. Acta 2020, 187, 186. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Han, T.; Li, X.; Sun, L.; Zhang, Y.; Zhang, Y. Colorimetric detection of kanamycin based on analyte-protected silver nanoparticles and aptamer-selective sensing mechanism. Anal. Chim. Acta 2015, 891, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Liu, X.; Qin, L.; Zhang, C.; Zhang, G.; Huang, D.; Cheng, M.; Xu, P.; Yi, H.; Huang, D. Chitosan-wrapped gold nanoparticles for hydrogen-bonding recognition and colorimetric determination of the antibiotic kanamycin. Microchim. Acta 2017, 184, 2097–2105. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, G.; Lai, C.; Huang, D.; Zhang, C.; Xua, P.; Hua, T.; Liu, X.; Cheng, M.; Liu, Y.; et al. A visual application of gold nanoparticles: Simple, reliable and sensitive detection of kanamycin based on hydrogen-bonding recognition. Sens. Actuator B Chem. 2017, 243, 946–954. [Google Scholar] [CrossRef]
- Gu, Z.F.; Fu, A.C.; Ye, L.; Kuerban, K.; Wang, Y.; Cao, Z.J. Ultrasensitive chemiluminescence biosensor for nuclease and bacterial determination based on hemin-encapsulated mesoporous silica nanoparticles. ACS Sens. 2019, 11, 2922–2929. [Google Scholar] [CrossRef]
- Wan, G.H.; Cui, H.; Zheng, H.-S.; Pang, Y.-Q.; Liu, L.-J.; Yu, X.-F. Flow-injection determination of streptomycin residues in milk using the luminol-periodate-Mn2+ chemiluminescence system. Luminescence 2006, 21, 36–42. [Google Scholar] [CrossRef]
- Deng, M.; Xu, S.; Chen, F. Enhanced chemiluminescence of the luminol-hydrogen peroxide system by BSA-stabilized Au nanoclusters as a peroxidase mimic and its application. Anal. Methods 2014, 6, 3117–3123. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Duan, W.; Li, F. A label-free, versatile and low-background chemiluminescence aptasensing strategy based on gold nanocluster catalysis combined with the separation of magnetic beads. Analyst 2018, 143, 709–714. [Google Scholar] [CrossRef]
- Sun, Y.; Han, R.; Dai, Y.; Zhu, X.; Liu, H.; Gao, D.; Luo, C.; Wang, X.; Wei, Q. Highly selective and sensitive streptomycin chemiluminescence sensor based on aptamer and g-quadruplex DNAzyme modified three-dimensional graphene composite. Sens. Actuator B Chem. 2019, 301, 127122. [Google Scholar] [CrossRef]
- Zheng, X.W.; Guo, Z.H.; Zhang, Z.J. Flow-injection electrogenerated chemiluminescence determination of isoniazid using luminol. Anal. Sci. 2001, 17, 1095–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Liu, H.; Zhang, H.; Chu, G.; Guo, Y.; Sun, X. Ultrasensitive electrochemiluminescence aptasensor for kanamycin detection based on silver nanoparticle-catalyzed chemiluminescent reaction between luminol and hydrogen peroxide. Sens. Actuator B Chem. 2020, 304, 127367. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Yin, G.; Zhang, Q.; Luo, J.; Wu, N. Aptamer-molecularly imprinted sensor base on electrogenerated chemiluminescence energy transfer for detection of lincomycin. Biosens. Bioelectron. 2017, 91, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shin, K.S. Surface-enhanced Raman scattering: A powerful tool for chemical identification. Anal. Sci. 2011, 27, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Knauer, M.; Ivleva, N.P.; Niessner, R.; Haisch, C. Optimized surface-enhanced Raman scattering (SERS) colloids for the characterization of microorganisms. Anal. Sci. 2010, 26, 761–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.Q.; Lim, C.; Chen, L.X.; Chon, H.; Choo, J.; Hong, J.; deMello, A.J. Surface-enhanced Raman scattering in nanoliter droplets: Towards high-sensitivity detection of mercury (II) ions. Anal. Bioanal. Chem. 2009, 394, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Huang, J.; Sun, Y.; Yin, M.; Hu, M.; Hu, X.; Zhang, Z.; Zhang, G. Utilization of a lateral flow colloidal gold immunoassay strip based on surface-enhanced Raman spectroscopy for ultrasensitive detection of antibiotics in milk. Spectroc. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 107–113. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 2019, 197, 151–158. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Ma, X.; Park, H.G.; Sim, S.J. Low-blinking SERS substrate for switchable detection of kanamycin. Sens. Actuator B Chem. 2019, 282, 765–773. [Google Scholar] [CrossRef]
- Ling, Y.; Fu, X.B.; Li, N.B.; Luo, H.Q. A label-free resonance rayleigh scattering sensor for detection of thrombin based on aptamer recognizing. Anal. Sci. 2018, 34, 881–886. [Google Scholar]
- Yan, S.; Lai, X.; Wang, Y.; Ye, N.; Xiang, Y. Label free aptasensor for ultrasensitive detection of tobramycin residue in pasteurized cow’s milk based on resonance scattering spectra and nanogold catalytic amplification. Food Chem. 2019, 295, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Liang, A.; Jiang, Z. A simple and selective resonance rayleigh scattering-energy transfer spectral method for determination of trace neomycin sulfate using cu2o particle as probe. Spectroc. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, C.; Sun, Y.; Shao, Y.; Cai, Y.; Zhang, Y.; Miao, J.; Miao, P. A colorimetric aptasensor for the antibiotics oxytetracycline and kanamycin based on the use of magnetic beads and gold nanoparticles. Microchim. Acta 2018, 185, 548. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Cai, G.; Yu, Z.; Tang, D. Glucose-loaded liposomes for amplified colorimetric immunoassay of streptomycin based on enzyme-induced iron(II) chelation reaction with phenanthroline. Sens. Actuator B Chem. 2018, 265, 174–181. [Google Scholar] [CrossRef]
- Zeng, R.; Tang, Y.; Zhang, L.; Luo, Z.; Tang, D. Dual-readout aptasensing of antibiotic residues based on gold nanocluster-functionalized mno2 nanosheets with target-induced etching reaction. J. Mater. Chem. B 2018, 6, 8071–8077. [Google Scholar] [CrossRef]
- Xu, C.; Ying, Y.; Ping, J. Colorimetric aggregation assay for kanamycin using gold nanoparticles modified with hairpin DNA probes and hybridization chain reaction-assisted amplification. Microchim. Acta 2019, 186, 448. [Google Scholar] [CrossRef]
- He, L.; Shen, Z.; Cao, Y.; Li, T.; Wu, D.; Dong, Y.; Gan, N. A microfluidic chip based ratiometric aptasensor for antibiotic detection in foods using stir bar assisted sorptive extraction and rolling circle amplification. Analyst 2019, 144, 2755–2764. [Google Scholar] [CrossRef]
- Ravikumar, A.; Panneerselvam, P. A novel fluorescent sensing platform based on metal-polydopamine frameworks for the dual detection of kanamycin and oxytetracycline. Analyst 2019, 144, 2337–2344. [Google Scholar]
- Yang, Q.; Hong, J.; Wu, Y.-X.; Cao, Y.; Wu, D.; Hu, F.; Gan, N. A multicolor fluorescence nanoprobe platform using two-dimensional metal organic framework nanosheets and double stirring bar assisted target replacement for multiple bioanalytical applications. ACS Appl. Mater. Interfaces 2019, 11, 41506–41515. [Google Scholar] [CrossRef]
- Youn, H.; Lee, K.; Her, J.; Jeon, J.; Mok, J.; So, J.I.; Shin, S.; Ban, C. Aptasensor for multiplex detection of antibiotics based on fret strategy combined with aptamer/graphene oxide complex. Sci. Rep. 2019, 9, 7659. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Jin, X.; Xing, Y.; Ni, G.; Peng, J. Construction of an NAND logic gate based on molecularly imprinted dual-emission quantum dot composites for the detection of antibiotics. Anal. Methods 2019, 11, 2033–2040. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, X.; Wei, D.; Huang, Z.; Cheng, S.; Chen, J. Chemiluminescence imaging immunoassay for multiple aminoglycoside antibiotics in cow milk. Int. J. Food Sci. Technol. 2020, 55, 119–126. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, J.; Sun, Y.; Deng, R.; Teng, M.; Li, Q.; Yang, Y.; Hu, X.; Zhang, Z.; Zhang, G. A SERS-based multiple immuno-nanoprobe for ultrasensitive detection of neomycin and quinolone antibiotics via a lateral flow assay. Microchim. Acta 2018, 185, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gan, N.; Hu, F.; Li, T.; Cao, Y.; Wu, D. Microchip electrophoresis array-based aptasensor for multiplex antibiotic detection using functionalized magnetic beads and polymerase chain reaction amplification. Sens. Actuator B Chem. 2018, 263, 568–574. [Google Scholar] [CrossRef]






| Name of AGs | Animals | Detection Object | MRLs (µg kg−1) | |||
|---|---|---|---|---|---|---|
| U.S. | E.U. | China | Japan | |||
| Streptomycin | Cattle | Muscle/fat/liver | 500 | 500 | 600 | 600 |
| Milk | - | 200 | 200 | 200 | ||
| Neomycin | Cattle/pork | Muscle/fat/liver | 1200/- | 500 | 500 | 500 |
| Milk | 150 | 1500 | 500 | 500 | ||
| Chicken | Egg | - | 500 | 500 | 500 | |
| Kanamycin | Cattle | Muscle/fat | - | 100 | - | 40 |
| liver | - | 600 | - | 40 | ||
| Milk | - | 150 | - | 400 | ||
| Pork | Muscle | - | 100 | - | ||
| fat | - | 100 | - | 100 | ||
| liver | - | 600 | - | 100 | ||
| Chicken | Egg | - | - | - | 500 | |
| Gentamicin | Cattle/pork | Muscle/fat | 100 | 50 | 100/400 | 100 |
| liver | 300 | 200 | 2000 | 2000 | ||
| Milk | - | 100 | 200 | 200 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Ji, Y.; Cao, Z. Recent Advances in Optical Detection of Aminoglycosides. Appl. Sci. 2020, 10, 6579. https://doi.org/10.3390/app10186579
Zhou Y, Ji Y, Cao Z. Recent Advances in Optical Detection of Aminoglycosides. Applied Sciences. 2020; 10(18):6579. https://doi.org/10.3390/app10186579
Chicago/Turabian StyleZhou, Yan, Yanwei Ji, and Zhijuan Cao. 2020. "Recent Advances in Optical Detection of Aminoglycosides" Applied Sciences 10, no. 18: 6579. https://doi.org/10.3390/app10186579
APA StyleZhou, Y., Ji, Y., & Cao, Z. (2020). Recent Advances in Optical Detection of Aminoglycosides. Applied Sciences, 10(18), 6579. https://doi.org/10.3390/app10186579