Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach
Abstract
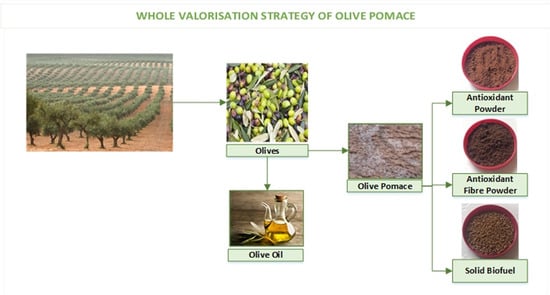
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Olive Pomace Samples
2.3. Fractionation of Olive Pomace
2.4. Chemical Composition Determination
2.4.1. Proximate Composition
2.4.2. Detergent Fibre
2.4.3. Cellulose, Hemicellulose and Lignin
2.4.4. Extractable Pectins
2.4.5. Soluble Sugars
2.5. Structural Characterisation
Chemical Groups and Bonding Arrangement of Constituents
2.6. Bioactive Characterisation
2.6.1. Free and Bound Phenolic Compounds
2.6.2. Antioxidant Activity
2.7. Energy Potential
2.8. Evaluation of the Potential Valorisation of Olive Pomace Using the Fractionation Approach in the Centre Region of Portugal: Case Study
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fractionation Approach
3.2. Proximate Composition
3.3. Detergent Fibre
3.4. Cellulose, Hemicellulose and Lignin
3.5. Pectins Quantification
3.6. Soluble Sugars
3.7. Infrared Spectroscopy
3.8. Bioactivity Characterisation
3.8.1. Total Phenolic Content and Antioxidant Activity of Free and Bound Phenolics
3.8.2. Identification of Phenolic Compounds
3.9. Energy Content
3.10. Evaluation of the Fractionation Valorisation Approach for Olive Pomace in the Centre Region of Portugal: Case Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ducom, G.; Gautier, M.; Pietraccini, M.; Tagutchou, J.-P.; Lebouil, D.; Gourdon, R. Comparative analyses of three olive mill solid residues from different countries and processes for energy recovery by gasification. Renew. Energy 2020, 145, 180–189. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 1–17. [Google Scholar] [CrossRef]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef]
- INE Previsões Agrícolas 31 de Janeiro 2020 Produção de Azeitona para Azeite com Máximo Histórico Ultrapassa as 940 mil Toneladas. 2020, pp. 1–5. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_destaques&DESTAQUESdest_boui=399592136&DESTAQUESmodo=2&xlang=pt (accessed on 28 February 2020).
- Nunes, M.A.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; Uceda-Rodríguez, M.; Cobo-Ceacero, C.J.; de Hoces, M.C.; MartínLara, M.Á.; Cotes-Palomino, T.; López García, A.B.; Martínez -García, C. Recycling of ‘alperujo’ (olive pomace) as a key component in the sintering of lightweight aggregates. J. Clean. Prod. 2019, 239, 118041. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutíerrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. New phenolic compounds hydrothermally extracted from the olive oil byproduct alperujo and their antioxidative activities. J. Agric. Food Chem. 2012, 60, 1175–1186. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of olive pomace by a green integrated approach applying sustainable extraction and membrane-assisted concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef]
- Salomone, R.; Ioppolo, G. Environmental impacts of olive oil production: A Life Cycle Assessment case study in the province of Messina (Sicily). J. Clean. Prod. 2012, 28, 88–100. [Google Scholar] [CrossRef]
- Galanakis, C.M. Olive fruit dietary fiber: Components, recovery and applications. Trends Food Sci. Technol. 2011, 22, 175–184. [Google Scholar] [CrossRef]
- Mata-Sánchez, J.; Pérez-Jiménez, J.A.; Díaz-Villanueva, M.J.; Serrano, A.; Núñez-Sánchez, N.; López-Giménez, F.J. Corrosive properties prediction from olive byproducts solid biofuel by near infrared spectroscopy. Energy Fuels 2014, 28, 5136–5143. [Google Scholar] [CrossRef]
- Mata Sánchez, J.; Pérez Jiménez, J.A.; Díaz Villanueva, M.J.; Serrano, A.; Núñez, N.; López Giménez, J. New techniques developed to quantify the impurities of olive stone as solid biofuel. Renew. Energy 2015, 78, 566–572. [Google Scholar] [CrossRef]
- AGAPA Evaluación de la Producción y Usos de los Subproductos de las Agroindustrias del Olivar en Andalucía. Cons. Agric. Pesca y Desarro. 2015. Available online: https://www.juntadeandalucia.es/agriculturaypesca/observatorio/servlet/FrontController?ec=default&action=DownloadS&table=11030&element=1585171&field=DOCUMENTO (accessed on 28 February 2020).
- Berbel, J.; Posadillo, A. Review and Analysis of Alternatives for the Valorisation of Agro-Industrial Olive Oil By-Products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Obtainment and characterization of a potential functional ingredient from olive. Int. J. Food Sci. Nutr. 2015, 66, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Ying, D.Y.; Hlaing, M.M.; Lerisson, J.; Pitts, K.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Physical properties and FTIR analysis of rice-oat flour and maize-oat flour based extruded food products containing olive pomace. Food Res. Int. 2017, 100, 665–673. [Google Scholar] [CrossRef]
- Delisi, R.; Ciriminna, R.; Arvati, S.; Meneguzzo, F.; Pagliaro, M. Olive biophenol integral extraction at a two-phase olive mill. J. Clean. Prod. 2018, 174, 1487–1491. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Álvarez-Parrilla, E.; de la Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Ereifej, K.; Alli, I. Distribution, antioxidant and characterisation of phenolic compounds in soybeans, flaxseed and olives. Food Chem. 2013, 139, 93–99. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC); Association of Official Analytical Chemists Inc.: Washington, DC, USA, 1990; ISBN 0-935584-42-0. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Procedures and Some Applications). Agric. Handb. 1970, 379, 5–11. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 Analytical Procedure-Determination of Structural Carbohydrates and Lignin in Biomass; NREL, 2012; Volume 17. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 18 March 2018).
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Issue Date 7/17/2005-42619; NREL, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42619.pdf (accessed on 18 March 2018).
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar]
- Campos, D.A.; Ribeiro, T.B.; Teixeira, J.A.; Pastrana, L.; Pintado, M.M. Integral Valorization of Pineapple (Ananas comosus L.) By-Products through a Green Chemistry Approach towards Added Value Ingredients. Foods 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, P.-J.; Huang, L.-X.; Zhang, C.; Zhang, Y.-L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Oliveira, A.L.; von Staszewski, M.; Pizones Ruiz-Henestrosa, V.M.; Pintado, M.; Pilosof, A.M.R. Impact of pectin or chitosan on bulk, interfacial and antioxidant properties of (+)-catechin and β-lactoglobulin ternary mixtures. Food Hydrocoll. 2016, 55, 119–127. [Google Scholar] [CrossRef]
- Monforte, A.R.; Martins, S.I.F.S.; Silva Ferreira, A.C. Strecker Aldehyde Formation in Wine: New Insights into the Role of Gallic Acid, Glucose, and Metals in Phenylacetaldehyde Formation. J. Agric. Food Chem. 2018, 66, 2459–2466. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Barros, A.S.; Silva Ferreira, A.C.; Silva, A.M.S. Influence of the temperature and oxygen exposure in red Port wine: A kinetic approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Acosta, M.; Arnao, M.B. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Rep. 2000, 5, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Pontes, R.; Romaní, A.; Michelin, M.; Domingues, L.; Teixeira, J.; Nunes, J. Comparative autohydrolysis study of two mixtures of forest and marginal land resources for co-production of biofuels and value-added compounds. Renew. Energy 2018, 128, 20–29. [Google Scholar] [CrossRef] [Green Version]
- INE. Estatísticas Agrícolas 2018; INE: Lisboa, Portugal, 2019; ISBN 9789892504957. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=383058708&PUBLICACOESmodo=2&xlang=pt (accessed on 16 March 2020).
- Venkata Mohan, S.; Dahiya, S.; Amulya, K.; Katakojwala, R.; Vanitha, T.K. Can circular bioeconomy be fueled by waste biorefineries—A closer look. Bioresour. Technol. Reports 2019, 7, 100277. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef]
- Uribe, E.; Lemus-Mondaca, R.; Vega-Gálvez, A.; Zamorano, M.; Quispe-Fuentes, I.; Pasten, A.; Di Scala, K. Influence of process temperature on drying kinetics, physicochemical properties and antioxidant capacity of the olive-waste cake. Food Chem. 2014, 147, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Sedlmeyer, F.B. Xylan as by-product of biorefineries: Characteristics and potential use for food applications. Food Hydrocoll. 2011, 25, 1891–1898. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; García, A.; Fernández-Bolaños, J. Novel pectin present in new olive mill wastewater with similar emulsifying and better biological properties than citrus pectin. Food Hydrocoll. 2015, 50, 237–246. [Google Scholar] [CrossRef]
- Gómez-González, S.; Ruiz-Jiménez, J.; Priego-Capote, F.; Luque De Castro, M.D. Qualitative and quantitative sugar profiling in olive fruits, leaves, and stems by gas chromatography-tandem mass spectrometry (GC-MS/MS) after ultrasound-assisted leaching. J. Agric. Food Chem. 2010, 58, 12292–12299. [Google Scholar] [CrossRef]
- Babich, O.; Dyshlyuk, L.; Noskova, S.; Sukhikh, S.; Prosekov, A.; Ivanova, S.; Pavsky, V. In vivo study of the potential of the carbohydrate-mineral complex from pine nut shells as an ingredient of functional food products. Bioact. Carbohydrates Diet. Fibre 2019, 18, 100185. [Google Scholar] [CrossRef]
- Fitch, C.; Keim, K.S. Position of the Academy of Nutrition and Dietetics: Use of Nutritive and Nonnutritive Sweeteners. J. Acad. Nutr. Diet. 2012, 112, 739–758. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Shahrestani, R.G. Subcritical water extraction of mannitol from olive leaves. J. Food Eng. 2009, 93, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Uncu, O.; Ozen, B.; Tokatli, F. Use of FTIR and UV–visible spectroscopy in determination of chemical characteristics of olive oils. Talanta 2019, 201, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Fakharedine, N.; El Hajjouji, H.; Ait Baddi, G.; Revel, J.C.; Hafidi, M. Chemical and spectroscopic analysis of organic matter transformation during aerobic digestion of olive-mill waste-waters. Process Biochem. 2006, 41, 398–404. [Google Scholar] [CrossRef]
- Cioffi, G.; Pesca, M.S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Carvalho, T.; Machado, N.; Barros, A. Kinetics of the Polyphenolic Content and Radical Scavenging Capacity in Olives through On-Tree Ripening. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Capriotti, A.L.; Cavaliere, C.; Crescenzi, C.; Foglia, P.; Nescatelli, R.; Samperi, R.; Laganà, A. Comparison of extraction methods for the identification and quantification of polyphenols in virgin olive oil by ultra-HPLC-QToF mass spectrometry. Food Chem. 2014, 158, 392–400. [Google Scholar] [CrossRef]
- Peralbo-Molina, Á.; Priego-Capote, F.; Luque De Castro, M.D. Tentative identification of phenolic compounds in olive pomace extracts using liquid chromatography-tandem mass spectrometry with a quadrupole- quadrupole-time-of-flight mass detector. J. Agric. Food Chem. 2012, 60, 11542–11550. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two- and three-phase centrifuge. LWT Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte. EFSA J. 2011, 9, 2033.
- Dang, H.; Meng, M.H.W.; Zhao, H.; Iqbal, J.; Dai, R.; Deng, Y.; Lv, F. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J. Nanopart. Res. 2014, 16, 2347. [Google Scholar]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Nieto Calvache, J.; Cueto, M.; Farroni, A.; de Escalada Pla, M.; Gerschenson, L.N. Antioxidant characterization of new dietary fiber concentrates from papaya pulp and peel (Carica papaya L.). J. Funct. Foods 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.F.; Camilo, C.J.; do Nascimento Silva, M.K.; de Freitas, T.S.; Ribeiro-Filho, J.; Coutinho, H.D.M. Vanillin selectively modulates the action of antibiotics against resistant bacteria. Microb. Pathog. 2017, 113, 265–268. [Google Scholar] [CrossRef]
- de Rezende Mudenuti, N.V.; de Camargo, A.C.; Shahidi, F.; Madeira, T.B.; Hirooka, E.Y.; Grossmann, M.V.E. Soluble and insoluble-bound fractions of phenolics and alkaloids and their antioxidant activities in raw and traditional chocolate: A comparative study. J. Funct. Foods 2018, 50, 164–171. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Topal, H.; Taner, T.; Naqvi, S.A.H.; Altınsoy, Y.; Amirabedin, E.; Ozkaymak, M. Exergy analysis of a circulating fluidized bed power plant co-firing with olive pits: A case study of power plant in Turkey. Energy 2017, 140, 40–46. [Google Scholar] [CrossRef]
- Vamvuka, D.; Kakaras, E. Ash properties and environmental impact of various biomass and coal fuels and their blends. Fuel Process. Technol. 2011, 92, 570–581. [Google Scholar] [CrossRef]
- Christoforou, E.; Fokaides, P.A. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef] [PubMed]




| Chemical Components | C-OP | L-OP | P-OP | ||||
|---|---|---|---|---|---|---|---|
| OM 1 | OM 2 | OM 1 | OM 2 | OM 1 | OM 2 | ||
| Proximate Compostion (g/100 g DW) | Moisture | 0.93 ± 0.01 b | 0.98 ± 0.07 b | 2.66 ± 0.31 a | 2.94 ± 0.01 a | 0.63 ± 0.04 b | 0.91 ± 0.04 b |
| Ash | 4.48 ± 0.09 c | 4.93 ± 0.09 d | 10.74 ± 0.21 b | 11.27 ± 0.18 a | 3.11 ± 0.19 e | 1.97 ± 0.03 f | |
| Crude Fibre | 35.90 ± 1.32 b | 31.94 ± 1.44 b | 0.13 ± 0.10 c | 0.09 ± 0.01 c | 54.54 ± 2.63 a | 54.08 ± 2.06 a | |
| Protein | 8.75 ± 0.13 a | 8.82 ± 0.15 a | 3.80 ± 0.24 d | 4.41 ± 0.12 c | 7.98 ± 0.18 b | 8.71 ± 0.12 a | |
| Fat | 15.61 ± 1.37 b | 20.04 ± 0.58 a | 2.68 ± 0.29 d | 5.56 ± 0.50 c | 14.99 ± 0.41 b | 21.34 ± 0.94 a | |
| Carbohydrates | 33.28 ± 2.37 b | 32.31 ± 1.29 b | 77.17 ± 0.88 a | 73.34 ± 1.12 a | 18.77 ± 2.87 c | 11.92 ± 1.89 d | |
| Detergent Fibre (g/100 g DW) | NDF | 46.48 ± 1.47 b,c | 40.76 ± 2.76 c | ND | ND | 53.29 ± 0.46 a,b | 59.28 ± 1.98 a |
| ADF | 31.06 ± 0.73 b | 25.34 ± 0.95 c | ND | ND | 33.51 ± 0.88 a,b | 36.66 ± 0.62 a | |
| Structural Carbohydrates (g/100 g DW) | Cellulose (as glucose) | 9.55 ± 0.38 a,b | 8.60 ± 0.54 b | ND | ND | 10.90 ± 1.26 a | 10.32 ± 0.68 a,b |
| Hemicellulose | 11.29 ± 0.50 a,b | 10.28 ± 0.25 b | ND | ND | 11.85 ± 0.72 a | 12.40 ± 0.94 a | |
| Xylose | 8.03 ± 0.26 b | 6.50 ± 0.21 b | ND | ND | 8.07 ± 0.51 a | 8.35 ± 0.69 a | |
| Arabinose | 0.36 ± 0.13 b | 0.83 ± 0.13 a | ND | ND | 0.61 ± 0.11 a,b | 1.70 ± 0.06 a | |
| Mannose | 1.02 ± 0.32 a | 1.06 ± 0.20 a | ND | ND | 1.05 ± 0.15 a | 1.26 ± 0.21 a | |
| Galactose | 1.88 ± 0.05 a | 1.79 ± 0.05 b | ND | ND | 2.12 ± 0.03 a | 2.07 ± 0.01 a | |
| Lignin | 43.95 ± 1.31 a | 42.48 ± 0.56 a | ND | ND | 43.38 ± 0.32 a | 45.72 ± 1.76 a | |
| Insoluble | 26.84 ± 0.76 a | 25.06 ± 1.69 a | ND | ND | 23.62 ± 0.94 a | 26.49 ± 1.53 a | |
| Soluble | 17.12 ± 0.76 a | 17.42 ± 1.21 a | ND | ND | 19.76 ± 0.63 a | 19.23 ± 0.24 a | |
| Pectins (g GUAE/100 g DW) | TSP | 3.23 ± 0.50 a | 2.92 ± 0.24 a | 0.69 ± 0.28 b | 1.33 ± 0.16 b | 3.37 ± 0.90 a | 2.64 ± 0.15 a |
| WSP | 0.64 ± 0.22 d,e | 1.00 ± 0.16 b | 0.77 ± 0.12 b,c | 1.32 ± 0.16 a | 0.49 ± 0.08 c,d | 0.46 ± 0.16 d | |
| CSP | 2.50 ± 0.34 a,b | 1.86 ± 0.26 b | ND | ND | 2.86 ± 0.81 a | 2.12 ± 0.10 a,b | |
| HSP | 0.09 ± 0.04 a | 0.17 ± 0.10 a | ND | ND | 0.02 ± 0.01 a | 0.06 ± 0.01 | |
| Soluble Sugars (g/100 g DW) | Total soluble sugars *1 | 6.56 ± 0.36 e | 9.36 ± 0.48 c | 19.01 ± 3.74 b | 28.78 ± 3.40 d | 2.36 ± 0.25 c | 4.41 ± 0.16 c |
| Glucose | 6.85 ± 1.42 b | 6.31 ± 0.61 b,c | 19.75 ± 2.00 c | 20.37 ± 1.74 c | 4.00 ± 0.90 b,c | 2.67 ± 0.61 c | |
| Mannitol | 7.16 ± 1.40 c,d | 10.55 ± 1.66 c | 21.10 ± 2.55 c | 32.37 ± 2.65 b | 4.81 ± 1.61 c | 4.08 ± 0.88 d | |
| Phenolic Compound | FPC | BPC | RT (min) | UV-Vis max | Formula | m/z exp | m/z theo | Error (mDa) | mSigma | Fragments | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxytyrosol and tyrosol derivatives | |||||||||||
| Dihydroxytyrosol | ✓ | ✓ | 1.9 | 279 | C8H10O4 | 169.0504 | 169.0506 | 0.3 | 5.1 | 151.0397; 123.0448 | [54] |
| Hydroxytyrosol glucoside | ✓ | ✓ | 3.9 | 278 | C14H20O8 | 315.1092 | 315.1088 | −0.4 | 16.3 | 315.1090; 153.0555; 123.0450 | [55] |
| Hydroxytyrosol | ✓ | ✓ | 4.3 | 281 | C8H10O3 | 153.0557 | 153.0555 | −0.2 | 0.9 | 153.0554; 123.0450 | (a) |
| Tyrosol glucoside | ✓ | ✘ | 8.3 | 275 | C14H20O7 | 299.1144 | 299.1136 | −0.2 | 15.1 | 299.1139; 119.0349; 101.0244, 89.0245 | [55] |
| Tyrosol | ✓ | ✘ | 12 | 276 | C8H10O2 | 137.0609 | 137.0608 | 0.5 | n.a. | 137.0603; 111.0084; 95.0510 | (a) |
| Secoiridoids and Derivatives | |||||||||||
| Oleoside | ✓ | ✓ | 6.2 | 270 | C16H22O11 | 389.1092 | 389.1089 | −0.3 | 5.6 | 389.1088;183.0664; 165.0560; 121.0656; 345.1195 | [55] |
| Verbascoside | ✓ | ✘ | 13.3 | 330 | C29H36O15 | 623.1990 | 623.1981 | −0.9 | 8.3 | 623.1983; 161.0244; 461.1665; 162.0276; 135.0451 | [55] |
| Caffeoyl-6′-secologanoside | ✓ | ✘ | 14.7 | 326 | C25H28O14 | 551.1416 | 551.1406 | 0 | 18.1 | 551.1416; 507.1504; 345.1193; 281.0673; 161.0245 | [55] |
| Oleuropein | ✓ | ✘ | 17.1 | 280 | C25H32O13 | 539.1761 | 539.1771 | 0.7 | 27.6 | 539.1764; 307.0828; 275.0931; 223.0613; 179.0566 | (a) |
| Comselogoside | ✓ | ✘ | 17.5 | 311 | C25H28O13 | 535.1462 | 535.1457 | −0.1 | 19.9 | 535.1465; 145.0296; 491.1558; 389.1093; 345.1197 | [55] |
| Flavonoids | |||||||||||
| Rutin | ✓ | ✓ | 10.7 | 355 | C27H30O16 | 609.1465 | 609.1461 | −0.1 | 18 | 609.1462; 300.0289 | (a) |
| Luteolin-7-O-glucoside | ✓ | ✓ | 11.6 | 349 | C21H20O11 | 447.0929 | 447.0933 | 0.8 | 20.5 | 447.0925; 285.0414 | (a) |
| Luteolin | ✓ | ✘ | 20.4 | 349 | C15H10O6 | 285.0414 | 285.0405 | −1 | 3.3 | 285.0414; 151.0037 | (a) |
| Quercetin | ✓ | ✘ | 20.5 | 342 | C15H10O7 | 301.0362 | 301.0354 | −0.5 | 4.5 | 301.0359; 151.0035; 178.9988; 121.0294 | (a) |
| Apigenin | ✓ | ✘ | 25.0 | 339 | C15H10O5 | 269.0461 | 269.0455 | −0.5 | 4.8 | 269.0461; 151.0035 | (a) |
| Phenolic Acids | |||||||||||
| Vanillin | ✘ | ✓ | 4.2 | 279 | C8H8O3 | 151.0400 | 151.0401 | 0.3 | 18.7 | 151.0397; 137.0235; 109.0290; 105.0341 | (a) |
| Hydroxybenzoic acid | ✘ | ✓ | 5.6 | C7H6O3 | 137.0241 | 137.0244 | 0.4 | 4 | 137.0241; 138.0280 | [54] | |
| Caffeic acid-3-glucoside | ✓ | ✓ | 5.8 | 277 | C15H18O9 | 341.0876 | 341.0878 | 0.2 | 9.5 | 341.0876; 179.0351; 135.0450 | [55] |
| Caffeic acid | ✓ | ✓ | 7.0 | 323 | C9H8O4 | 179.0350 | 179.0350 | 0.0 | 7.5 | 179.0350; 135.0448 | (a) |
| Coumaric acid | ✓ | ✓ | 9.0 | 309 | C9H8O3 | 163.0397 | 163.0401 | 0.3 | 19.6 | 163.0397; 119.0499 | (a) |
| Ferulic acid | ✘ | ✓ | 10.4 | 323 | C10H10O4 | 193.0509 | 193.0506 | −0.3 | 18.6 | 193.0504; 178.0268; 134.0370 | (a) |
| Phenolic Compound | C-OP | L-OP | P-OP | ||||
|---|---|---|---|---|---|---|---|
| OM 1 | OM 2 | OM 1 | OM 2 | OM 1 | OM 2 | ||
| Hydroxytyrosol | Free | 207.08 ± 13.95 c | 173.67 ± 13.68 c | 573.43 ± 59.62 a | 504.73 ± 27.67 b | 81.62 ± 20.98 d | 26.54 ± 8.35 e |
| Bound | 17.35 ± 6.03 c | 16.44 ± 4.70 c | 78.51 ± 8.70 a | 15.99 ± 1.53 c | 43.14 ± 9.85 b | 11.93 ± 3.69 c | |
| Tyrosol | Free | 51.21 ± 3.01 b | 65.89 ± 6.91 a | ND | ND | 35.48 ± 1.87 c | 20.75 ± 3.48 d |
| Protocatechuic acid | Bound | 10.30 ± 1.17 b | 8.38 ± 1.02 b | ND | ND | 15.73 ± 1.32 a | 10.09 ± 1.03 b |
| Caffeic acid | Free | 21.92 ± 1.71 a | 8.79 ± 1.25 c | 23.63 ± 3.10 a | 15.14 ± 0.85 b | 14.01 ± 0.58 b | 0.51 ± 0.12 d |
| Bound | 25.15 ± 2.15 b | 16.61 ± 3.25 c,d | 40.71 ± 5.23 a | 23.10 ± 1.49 b,c | 34.76 ± 3.48 a | 13.47 ± 1.87 d | |
| Vanillin | Bound | 1.39 ± 0.23 b | 0.85 ± 0.20 c | ND | ND | 1.63 ± 0.18 a,b | 1.79 ± 0.26 a |
| p-Coumaric acid | Free | 7.41 ± 0.58 b | 8.30 ± 0.97 b | 8.04 ± 1.17 b | 15.75 ± 1.80 a | 6.46 ± 0.16 b | 1.40 ± 0.13 c |
| Bound | 8.69 ± 3.50 d | 14.47 ± 2.85 b | 13.43 ± 2.60 b,c | 19.47 ± 0.45 a | 9.64 ± 0.64 c,d | 15.64 ± 0.80 a,b | |
| Rutin | Free | ND | 30.85 ± 2.71 a | ND | ND | ND | 16.49 ± 0.93 b |
| Luteolin-7-O-glucoside | Free | ND | 10.51 ± 0.77 a | ND | ND | ND | 10.31 ± 1.09 a |
| Luteolin | Free | 18.40 ± 0.51 c | 44.47 ± 2.98 a | ND | ND | 22.34 ± 0.66 b | 45.26 ± 1.47 a |
| Quercitin | Free | 3.22 ± 0.34 a,b | 3.45 ± 0.46 a | ND | ND | 2.80 ± 0.36 b | 0.91 ± 0.24 c |
| Total | Free | 290.18 ± 15.51 c | 346.03 ± 24.37 d | 581.47 ± 60.70 a | 520.47 ± 29.17 b | 152.45 ± 22.0 e | 127.66 ± 31.43 e |
| 77% | 84% | 84% | 92% | 58% | 72% | ||
| Bound | 62.87 ± 8.26 c | 56.75 ± 10.99 c | 132.64 ± 0.93 a | 58.55 ± 2.41 c | 97.17 ± 18.98 b | 51.41 ± 8.49 c | |
| 17% | 13% | 19% | 10% | 37% | 28% | ||
| High Calorific Value (MJ/Kg Dry Weight) | |||||||
|---|---|---|---|---|---|---|---|
| C-OP | P + S-OP | P-OP | S-OP | ||||
| OM 1 | OM 2 | OM 1 | OM 2 | OM 1 | OM 2 | OM 1 | OM 2 |
| 20.57 ± 0.03 c,d | 21.67 ± 0.20 b | 20.21 ± 0.05 d | 20.86 ± 0.11 c | 21.52 ± 0.12 b | 22.21 ± 0.01 a | 18.94 ± 0.00 d | 18.65 ± 0.00 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Appl. Sci. 2020, 10, 6785. https://doi.org/10.3390/app10196785
Ribeiro TB, Oliveira AL, Costa C, Nunes J, Vicente AA, Pintado M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Applied Sciences. 2020; 10(19):6785. https://doi.org/10.3390/app10196785
Chicago/Turabian StyleRibeiro, Tânia B., Ana L. Oliveira, Cristina Costa, João Nunes, António A. Vicente, and Manuela Pintado. 2020. "Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach" Applied Sciences 10, no. 19: 6785. https://doi.org/10.3390/app10196785
APA StyleRibeiro, T. B., Oliveira, A. L., Costa, C., Nunes, J., Vicente, A. A., & Pintado, M. (2020). Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Applied Sciences, 10(19), 6785. https://doi.org/10.3390/app10196785