Integrated System Technology of POME Treatment for Biohydrogen and Biomethane Production in Malaysia
Abstract
:1. Introduction
POME Treatment Systems in Malaysia
2. Biogas Production from POME
Challenges Using POME Wastewater
3. Biohydrogen Production via Dark Fermentation (DF)
3.1. Dark Fermentative Bacteria
3.1.1. Obligate Anaerobic Bacteria
3.1.2. Mixed Cultures
3.1.3. Thermophiles
3.2. Biochemistry of Dark Fermentation
4. Biomethane Production via Anaerobic Digestion (AD)
Biochemistry of Anaerobic Digestion
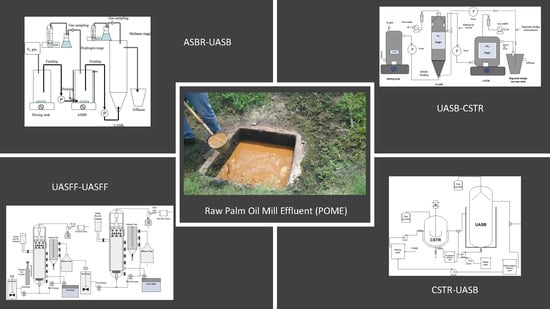
5. An Integrated System as an Innovative Approach for Biohydrogen, Biomethane Production and Wastewater Treatment
6. Importance of Biohydrogen and Biomethane
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Jung, K.W.; Moon, C.; Cho, S.K.; Kim, S.H.; Shin, H.S.; Kim, D.H. Conversion of organic solid waste to hydrogen and methane by two-stage fermentation system with reuse of methane fermenter effluent as diluting water in hydrogen fermentation. Bioresour. Technol. 2013, 139, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Kim, D.H.; Kim, S.H.; Shin, H.S. Bioreactor design for continuous dark fermentative hydrogen production. Bioresour. Technol. 2011, 102, 8612–8620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Fang, Z.; Chin, S.X.; Tian, X.F.; Su, T.C. Biohydrogen Production from Hydrolysates of Selected Tropical Biomass Wastes with Clostridium Butyricum. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, Y.C. A bench scale study of fermentative hydrogen and methane production from food waste in integrated two-stage process. Int. J. Hydrogen Energy 2009, 34, 245–254. [Google Scholar] [CrossRef]
- Lay, J.J.; Tsai, C.J.; Huang, C.C.; Chang, J.J.; Chou, C.H.; Fan, K.S.; Chang, J.I.; Hsu, P.C. Influences of pH and hydraulic retention time on anaerobes converting beer processing wastes into hydrogen. Water Sci. Technol. 2005, 52, 123–129. [Google Scholar] [CrossRef]
- Wilkie, A.C. Biomethane from biomass, biowaste, and biofuels. In Bioenergy; ASM Press: Washington, DC, USA, 2008; pp. 195–205. [Google Scholar]
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR Article in Waste Management. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef]
- Wilkie, A.C. Anaerobic Digestion: Biology and Benefits. In Dairy Manure Management: Treatmnet, Handling, and Community Relations; Cornell University: Ithaca, NY, USA, 2005; pp. 63–72. [Google Scholar]
- Alam, A.F.; Er, A.C.; Begum, H. Malaysian oil palm industry: Prospect and problem. J. Food Agric. Environ. 2015, 13, 143–148. [Google Scholar]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.; Anuar, N. A holistic approach to managing palm oil mill effluent (POME): Biotechnological advances in the sustainable reuse of POME. Biotechnol. Adv. 2009, 27, 40–52. [Google Scholar] [CrossRef]
- Basiron, Y. Palm oil production through sustainable plantations. Eur. J. Lipid Sci. Technol. 2007, 109, 289–295. [Google Scholar] [CrossRef]
- Loh, S.K.; Choo, Y.M. Prospect, challenges and opportunities on biofuels in Malaysia. In Advances in Biofuels; Pogaku, R., Sarbatly, R., Eds.; Springer: Boston, MA, USA, 2013; pp. 3–14. [Google Scholar]
- Hosseini, S.E.; Wahid, M.A. Feasibility study of biogas production and utilization as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2013, 19, 454–462. [Google Scholar] [CrossRef]
- Loh, S.K.; Lai, M.E.; Ngatiman, M.; Lim, W.S.; Choo, Y.M.; Zhang, Z.; Salimon, J. Zero discharge treatment technology of Palm Oil Mill Effluent. J. Oil Palm Res. 2014, 25, 273–281. [Google Scholar]
- Wong, Y.S.; Kadir, M.O.A.B.; Teng, T.T. Biological kinetics evaluation of anaerobic stabilization pond treatment of palm oil mill effluent. Bioresour. Technol. 2009, 100, 4969–4975. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, A.A.; Mirghorayshi, M. Effect of Temperature on the Performance of an Up-flow Anaerobic Sludge Fixed Film (UASFF) Bioreactor Treating Palm Oil Mill Effluent (POME). Waste Biomass Valorization 2019, 10, 349–355. [Google Scholar] [CrossRef]
- Zainal, B.S.; Akhbari, A.; Zinatizadeh, A.A.; Mohammadi, P.; Danaee, M.; Mohd, N.S.; Ibrahim, S. UASFF start-up for biohydrogen and biomethane production from treatment of Palm Oil Mill Effluent. Int. J. Hydrogen Energy 2018, 44, 20725–20737. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A review on anaerobic—Aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Nurul Adela, B.; Muzzammil, N.; Loh, S.K.; Choo, Y.M. Characteristics of palm oil mill effluent (Pome) in an anaerobic biogas digester. Asian J. Microbiol. Biotechnol. Environ. Sci. 2014, 16, 225–231. [Google Scholar]
- Taha, M.R.; Ibrahim, A.H. COD removal from anaerobically treated palm oil mill effluent (AT-POME) via aerated heterogeneous Fenton process: Optimization study. J. Water Process Eng. 2014, 1, 8–16. [Google Scholar] [CrossRef]
- Loh, S.K.; Nasrin, A.B.; Mohamad Azri, S.; Nurul Adela, B.; Muzzammil, N.; Daryl Jay, T.; Stasha Eleanor, R.A.; Lim, W.S.; Choo, Y.M.; Kaltschmitt, M. First Report on Malaysia’ s experiences and development in biogas capture and utilization from palm oil mill effluent under the Economic Transformation Programme: Current and future perspectives. Renew. Sustain. Energy Rev. 2017, 74, 1257–1274. [Google Scholar] [CrossRef]
- Akhbari, A.; Akbar, A.; Vafaeifard, M.; Mohammadi, P.; Zainal, B.S.; Ibrahim, S. Effect of operational variables on biological hydrogen production from palm oil mill effluent by dark fermentation using response surface methodology. Desalin. Water Treat. 2018, 23169, 1–13. [Google Scholar] [CrossRef]
- Tan, H.M.; Lew, J.C.S.; Gouwanda, D.; Poh, P.E. Fuzzy Logic Modelling for Thermophilic Anaerobic Digestion of Palm Oil Mill Effluent (POME) Treatment. In Proceedings of the 2017 4th International Conference on Industrial Engineering and Applications (ICIEA), Nagoya, Japan, 21–23 April 2017. [Google Scholar]
- Ahmad, A.L.; Chong, M.F.; Bhatia, S. A comparative study on the membrane based palm oil mill effluent (POME) treatment plant. J. Hazard. Mater. 2009, 171, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, A.S.; Hock, L.S.; Yusof, M.Z.M.; Rahman, N.A.A.; Shah, U.K.M.; Hassan, M.A.; Wakisaka, M.; Sakai, K.; Shirai, Y. Effects of palm oil mill effluent (POME) anaerobic sludge from 500 m3 of closed anaerobic methane digested tank on pressed-shredded empty fruit bunch (EFB) composting process. Afr. J. Biotechnol. 2010, 9, 2427–2436. [Google Scholar]
- Khemkhao, M.; Techkarnjanaruk, S.; Phalakornkule, C. Effect of chitosan on reactor performance and population of specific methanogens in a modified CSTR treating raw POME. Biomass Bioenergy 2016, 86, 11–20. [Google Scholar] [CrossRef]
- Malaysian Palm Oil Board (MPOB) Oil Palm & the Environment. Available online: http://mpob.gov.my/en/palm-info/environment/520-achievements (accessed on 9 December 2018).
- Nahrul Hayawin, Z.; Nor Faizah, J.; Ropandi, M.; Astimar, A.A. A review on the development of palm oil mill effluent (POME) final discharge polishing treatments. J. Oil Palm Res. 2017, 29, 528–540. [Google Scholar]
- Habib, M.A.B.; Yusoff, F.M.; Phang, S.M.; Ang, K.J.I.; Mohamed, S. Nutritional values of chironomid larvae grown in palm oil mill effluent and algal culture. Aquaculture 1997, 158, 95–105. [Google Scholar] [CrossRef]
- Rupani, P.F.; Singh, R.P.; Ibrahim, H.; Esa, N. Review of current palm oil mill effluent (POME) treatment methods: Vermicomposting as a sustainable practice. World Appl. Sci. J. 2010, 10, 1190–1201. [Google Scholar]
- Vijaya, S.; Ma, A.N.; Choo, Y.M. Capturing Biogas: A means to reduce green house gas emissions for the production of crude palm oil. Am. J. Geosci. 2010, 1, 1–6. [Google Scholar] [CrossRef]
- Chin, M.J.; Poh, P.E.; Tey, B.T.; Chan, E.S.; Chin, K.L. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev. 2013, 26, 717–726. [Google Scholar] [CrossRef]
- Poh, P.E.; Chong, M.F. Development of anaerobic digestion methods for palm oil mill effluent (POME) treatment. Bioresour. Technol. 2009, 100, 1–9. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ismail, S.; Bhatia, S. Water recycling from palm oil mill effluent (POME) using membrane technology. Desalination 2003, 157, 87–95. [Google Scholar] [CrossRef]
- Wang, J.; Mahmood, Q.; Qiu, J.P.; Li, Y.S.; Chang, Y.S.; Li, X.D. Anaerobic treatment of palm oil mill effluent in pilot-scale anaerobic EGSB reactor. BioMed Res. Int. 2015, 2015, 398028. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.M.; Abdul Raman, A.A. Trend and current practices of palm oil mill effluent polishing: Application of advanced oxidation processes and their future perspectives. J. Environ. Manag. 2017, 198, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Khemkhao, M.; Nuntakumjorn, B.; Techkarnjanaruk, S. Effect of chitosan on UASB treating POME during a transition from mesophilic to thermophilic conditions. Bioresour. Technol. 2011, 102, 4674–4681. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.J.; Chong, M.F.; Law, C.L. Optimization on thermophilic aerobic treatment of anaerobically digested palm oil mill effluent (POME). Biochem. Eng. J. 2011, 55, 193–198. [Google Scholar] [CrossRef]
- Najafpour, G.D.; Zinatizadeh, A.A.L.; Mohamed, A.R.; Hasnain Isa, M.; Nasrollahzadeh, H. High-rate anaerobic digestion of palm oil mill effluent in an upflow anaerobic sludge-fixed film bioreactor. Process Biochem. 2006, 41, 370–379. [Google Scholar] [CrossRef]
- Borja, R.; Banks, C.J.; Sinchez, E. Anaerobic treatment of palm oil mill effluent in a two-stage up-flow anaerobic sludge blanket (UASB) system. J. Biotechnol. 1996, 45, 125–135. [Google Scholar] [CrossRef]
- Mohammed, R.R.; Ketabachi, M.R.; McKay, G.; Ketabchi, M.R.; McKay, G. Combined magnetic field and adsorption process for treatment of biologically treated palm oil mill effluent (POME). Chem. Eng. J. 2014, 243, 31–42. [Google Scholar] [CrossRef]
- Mun, Y.W. Production of Methane from Palm Oil Mill Effluent by Using Ultrasonicated Membrane Anaerobic System (UMAS). Bachelor’s Thesis, Universiti Malaysia Pahang, Pekan, Malaysia, 2012; pp. 1–23. [Google Scholar]
- Najafpour, G.; Yieng, H.A.; Younesi, H.; Zinatizadeh, A. Effect of organic loading on performance of rotating biological contactors using Palm Oil Mill effluents. Process Biochem. 2005, 40, 2879–2884. [Google Scholar] [CrossRef]
- Wang, J.; Mahmood, Q.; Qiu, J.P.; Li, Y.S.; Chang, Y.S.; Chi, L.N.; Li, X.D. Zero discharge performance of an industrial pilot-scale plant treating palm oil mill effluent. Biomed Res. Int. 2015, 2015, 617861. [Google Scholar] [CrossRef]
- Choi, W.-H.; Shin, C.-H.; Son, S.-M.; Ghorpade, P.A.; Kim, J.-J.; Park, J.-Y. Anaerobic treatment of palm oil mill effluent using combined high-rate anaerobic reactors. Bioresour. Technol. 2013, 141, 138–144. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ismail, S.; Bhatia, S. Membrane treatment for palm oil mill effluent: Effect of transmembrane pressure and crossflow velocity. Desalination 2005, 179, 245–255. [Google Scholar] [CrossRef]
- Nahrul Hayawin, Z.; Astimar, A.A.; Idris, J.; Nor Faizah, J.; Ropandi, M.; Ibrahim, M.F.; Hassan, M.A.; Abd-Aziz, S. Reduction of POME final discharge residual using activated bioadsorbent from oil palm kernel shell. J. Clean. Prod. 2018, 182, 830–837. [Google Scholar]
- Athirah, W.N.; Hamdan, W.M.; Haan, Y.; Wahab Mohammad, A. Sustainable Approach in Palm Oil Industry-Green Synthesis of Palm Oil Mill Effluent Based Graphene Sand Composite (P-GSC) for Aerobic Palm Oil Mill Effluent Treatment (Pendekatan Mampan dalam Industri Minyak Kelapa Sawit-Sintesis Komposit Pasir Grafin (P-. J. Kejuruter. SI 2018, 1, 11–20. [Google Scholar]
- Ohimain, E.I.; Izah, S.C. A review of biogas production from palm oil mill effluents using different configurations of bioreactors. Renew. Sustain. Energy Rev. 2016, 70, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Igwe, J.C.; Onyegbado, C.C. A Review of Palm Oil Mill Effluent (POME) Water Treatment. Glob. J. Environ. Res. 2007, 1, 54–62. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Value-added utilization of oil palm ash: A superior recycling of the industrial agricultural waste. J. Hazard. Mater. 2009, 172, 523–531. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Abd-Aziz, S.; Ariffin, H.; Rahman, N.A.; Phang, L.Y.; Hassan, M.A. Comamonas sp. EB172 isolated from digester treating palm oil mill effluent as potential polyhydroxyalkanoate (PHA) producer. Afr. J. Biotechnol. 2008, 7, 4118–4121. [Google Scholar]
- Basri, M.F.; Yacob, S.; Hassan, M.A.; Shirai, Y.; Wakisaka, M.; Zakaria, M.R.; Phang, L.Y. Improved biogas production from palm oil mill effluent by a scaled-down anaerobic treatment process. World J. Microbiol. Biotechnol. 2010, 26, 505–514. [Google Scholar] [CrossRef]
- Mamimin, C.; Chaikitkaew, S.; Niyasom, C.; Kongjan, P.; Sompong, O. Effect of operating parameters on process stability of continuous biohydrogen production from palm oil mill effluent under thermophilic condition. Energy Procedia 2015, 79, 815–821. [Google Scholar] [CrossRef] [Green Version]
- Mamimin, C.; Prasertsan, P.; Kongjan, P.; Sompong, O. Effects of volatile fatty acids in biohydrogen effluent on biohythane production from palm oil mill effluent under thermophilic condition. Electron. J. Biotechnol. 2017, 29, 78–85. [Google Scholar] [CrossRef]
- Yossan, S.; Sompong, O.; Prasertsan, P. Effect of initial pH, nutrients and temperature on hydrogen production from palm oil mill effluent using thermotolerant consortia and corresponding microbial communities. Int. J. Hydrogen Energy 2012, 37, 13806–13814. [Google Scholar] [CrossRef]
- Mohammadi, P.; Ibrahim, S.; Annuar, M.S.M.; Khashij, M.; Mousavi, S.A.; Zinatizadeh, A. Optimization of fermentative hydrogen production from palm oil mill effluent in an up-flow anaerobic sludge blanket fixed film bioreactor. Sustain. Environ. Res. 2017, 27, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, C.; Lu, Y.; Wu, X.; Wang, L.; Wang, L.; Han, B.; Xing, X.H. States and challenges for high-value biohythane production from waste biomass by dark fermentation technology. Bioresour. Technol. 2013, 135, 292–303. [Google Scholar] [CrossRef]
- Mohammadi, P.; Ibrahim, S.; Mohamad Annuar, S.M. Comparative study on the effect of various pretreatment methods on the enrichment of hydrogen producing bacteria in anaerobic granulated sludge from brewery wastewater. Korean J. Chem. Eng. 2012, 29, 1347–1351. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Babu, V.L.; Sarma, P.N. Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Bioresour. Technol. 2008, 99, 59–67. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Li, B.; Wang, Y.; Liu, S. Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int. J. Hydrogen Energy 2006, 31, 2147–2157. [Google Scholar] [CrossRef]
- Khemkhao, M.; Nuntakumjorn, B.; Techkarnjanaruk, S.; Phalakornkule, C. UASB performance and microbial adaptation during a transition from mesophilic to thermophilic treatment of palm oil mill effluent. J. Environ. Manag. 2012, 103, 74–82. [Google Scholar] [CrossRef]
- Ahmad, A.; Buang, A.; Bhat, A.H.H. Renewable and sustainable bioenergy production from microalgal co-cultivation with palm oil mill effluent (POME): A review. Renew. Sustain. Energy Rev. 2016, 65, 214–234. [Google Scholar] [CrossRef]
- Norfadilah, N.; Raheem, A.; Harun, R. Bio-hydrogen production from palm oil mill effluent (POME): A preliminary study. Int. J. Hydrogen Energy 2016, 41, 11960–11964. [Google Scholar] [CrossRef]
- Mohammadi, P.; Ibrahim, S.; Mohamad Annuar, M.S.; Law, S.; Suf, M.; Annuar, M.; Law, S. Effects of different pretreatment methods on anaerobic mixed microflora for hydrogen production and COD reduction from palm oil mill effluent. J. Clean. Prod. 2011, 19, 1654–1658. [Google Scholar] [CrossRef] [Green Version]
- Han, S.K.; Shin, H.S. Biohydrogen production by anaerobic fermentation of food waste. Int. J. Hydrogen Energyy 2004, 29, 569–577. [Google Scholar] [CrossRef]
- Guwy, A.J.; Hawkes, F.R.; Hawkes, D.L.; Rozzi, A.G. Hydrogen production in a high rate fluidised bed anaerobic digester. Water Res. 1997, 31, 1291–1298. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.Y.; Lin, M.C. Acid–base enrichment enhances anaerobic hydrogen production process. Appl. Microbiol. Biotechnol. 2002, 58, 224–228. [Google Scholar]
- Shaw, A.J.; Jenney, F.E.; Adams, M.W.W.; Lynd, L.R. End-product pathways in the xylose fermenting bacterium, Thermoanaerobacterium saccharolyticum. Enzyme Microb. Technol. 2008, 42, 453–458. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Z.T.; Zhang, X. Biohydrogen production from starch wastewater and application in fuel cell. Int. J. Hydrogen Energy 2010, 35, 2949–2952. [Google Scholar] [CrossRef]
- Van De Werken, H.J.G.; Verhaart, M.R.A.; VanFossen, A.L.; Willquist, K.; Lewis, D.L.; Nichols, J.D.; Goorissen, H.P.; Mongodin, E.F.; Nelson, K.E.; Van Niel, E.W.J.; et al. Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl. Environ. Microbiol. 2008, 74, 6720–6729. [Google Scholar] [CrossRef] [Green Version]
- Sompong, O.; Prasertsan, P.; Intrasungkha, N.; Dhamwichukorn, S.; Birkeland, N.K. Improvement of biohydrogen production and treatment efficiency on palm oil mill effluent with nutrient supplementation at thermophilic condition using an anaerobic sequencing batch reactor. Enzyme Microb. Technol. 2007, 41, 583–590. [Google Scholar]
- Sompong, O.; Prasertsan, P.; Karakashev, D.; Angelidaki, I. Thermophilic fermentative hydrogen production by the newly isolated Thermoanaerobacterium thermosaccharolyticum PSU-2. Int. J. Hydrogen Energy 2008, 33, 1204–1214. [Google Scholar]
- Van Niel, E.W.J.; Budde, M.A.W.; De Haas, G.G.; Van der Wal, F.J.; Claassen, P.A.M.; Stams, A.J.M. Distinctive properties of high hydrogen producing extreme thermophiles, Caldicellulosiruptor saccharolyticus and Thermotoga elÿi. Int. J. Hydrogen Energy 2002, 27, 1391–1398. [Google Scholar] [CrossRef]
- Schroder, C.; Selig, M.; Schonheit, P. Glucose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic eubacterium Thermotoga maritima: Involvement of the Embden-Meyerhof pathway. Arch. Microbiol. 1994, 161, 460–470. [Google Scholar]
- Balachandar, G.; Khanna, N.; Das, D. Biohydrogen Production from Organic Wastes by Dark Fermentation, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444595553. [Google Scholar]
- McCord, J.M.; Keele, B.B.; Fridovich, I. An enzyme-based theory of obligate anaerobiosis: The physiological function of superoxide dismutase. Proc. Natl. Acad. Sci. USA 1971, 68, 1024–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeikus, J.G. The Biology of Methanogenic Bacteria. Bacteriol. Rev. 1977, 41, 514–541. [Google Scholar] [CrossRef] [PubMed]
- Kosaku, U.; Rabinowitz, J.C. Pyruvate-ferredoxin oxidoreductase. J. Biol. Chem. 1970, 246, 3111–3119. [Google Scholar]
- Mohan, S.V.; Pandey, A. Biohydrogen production: An introduction. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–24. ISBN 9780444595553. [Google Scholar]
- Khanna, N.; Kotay, S.M.; Gilbert, J.J.; Das, D. Improvement of biohydrogen production by Enterobacter cloacae IIT-BT 08 under regulated pH. J. Biotechnol. 2011, 152, 9–15. [Google Scholar] [CrossRef]
- Seghezzo, L. Anaerobic Treatment of Domestic Wastewater in Subtropical Regions; Wageningen University: Wageningen, The Netherlands, 2004. [Google Scholar]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Renewable and sustainable bioenergies production from palm oil mill effluent (POME): Win-win strategies toward better environmental protection. Biotechnol. Adv. 2011, 29, 124–141. [Google Scholar] [CrossRef]
- Wong, Y.S.; Teng, T.T.; Ong, S.A.; Norhashimah, M.; Rafatullah, M.; Lee, H.C. Anaerobic acidogenesis biodegradation of palm oil mill effluent using suspended closed anaerobic bioreactor (SCABR) at mesophilic temperature. Procedia Environ. Sci. 2013, 18, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P.; Sopian, K. Production of biogas and performance evaluation of existing treatment processes in palm oil mill effluent (POME). Renew. Sustain. Energy Rev. 2015, 42, 1260–1278. [Google Scholar] [CrossRef]
- Mansor, M.F.; Jahim, J.M.; Mumtaz, T.; Rahman, R.A.; Mutalib, S.A. Development of a methane-free, continuous biohydrogen production system from palm oil mill effluent (POME) in CSTR. J. Eng. Sci. Technol. 2016, 11, 1174–1182. [Google Scholar]
- Mohammadi, P.; Ibrahim, S.; Mohamad Annuar, M.S. High-rate fermentative hydrogen production from palm oil mill effluent in an up-flow anaerobic sludge blanket-fixed film reactor. Chem. Eng. Res. Des. 2014, 92, 1811–1817. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Alkasrawi, M. Process enhancement of hydrogen and methane production from palm oil mill effluent using two-stage thermophilic and mesophilic fermentation. Int. J. Hydrogen Energy 2016, 41, 12888–12898. [Google Scholar] [CrossRef] [Green Version]
- Suksong, W.; Kongjan, P.; Sompong, O. Biohythane Production from co-digestion of palm oil mill effluent with solid residues by two-stage solid state anaerobic digestion process. Energy Procedia 2015, 79, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Mamimin, C.; Singkhala, A.; Kongjan, P.; Suraraksa, B.; Prasertsan, P.; Imai, T.; Sompong, O. Two-stage thermophilic fermentation and mesophilic methanogen process for biohythane production from palm oil mill effluent. Int. J. Hydrogen Energy 2015, 40, 6319–6328. [Google Scholar] [CrossRef]
- Sompong, O.; Suksong, W.; Promnuan, K.; Thipmunee, M.; Mamimin, C.; Prasertsan, P. Two-stage thermophilic fermentation and mesophilic methanogenic process for biohythane production from palm oil mill effluent with methanogenic effluent recirculation for pH control. Int. J. Hydrogen Energy 2016, 41, 21702–21712. [Google Scholar]
- Zinatizadeh, A.A.L.; Mohamed, A.R.; Abdullah, A.Z.; Mashitah, M.D.; Isa, M.H.; Najafpour, G.D. Process modeling and analysis of palm oil mill effluent treatment in an up-flow anaerobic sludge fixed film bioreactor using response surface methodology (RSM). Water Res. 2006, 40, 3193–3208. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.L.; Jaafar, A.B. POME Biogas capture, upgrading and utilization. Palm Oil Eng. Bull. 2006, 78, 11–17. [Google Scholar]
- Kongjan, P.; Sompong, O.; Angelidaki, I. Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour. Technol. 2011, 102, 4028–4035. [Google Scholar] [CrossRef]
- Kanchanasuta, S.; Sillaparassamee, O. Enhancement of hydrogen and methane production from co-digestion of palm oil decanter cake and crude glycerol using two stage thermophilic and mesophilic fermentation. Int. J. Hydrogen Energy 2017, 42, 3440–3446. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Effect of hydraulic retention time (HRT) on the anaerobic co-digestion of agro-industrial wastes in a two-stage CSTR system. Bioresour. Technol. 2014, 167, 407–415. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Sohaili, J. Effect of organic loading rate on hydrogen (H2) and methane (CH4) production in two-stage fermentation under thermophilic conditions using palm oil mill effluent (POME). Energy Sustain. Dev. 2016, 34, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Md Din, M.F.; Taib, S.M.; Nasrullah, M.; Sakinah, M.; Wahid, Z.A.; Kamyab, H.; Chelliapan, S.; Rezania, S.; Singh, L. Accelerated two-stage bioprocess for hydrogen and methane production from palm oil mill effluent using continuous stirred tank reactor and microbial electrolysis cell. J. Clean. Prod. 2019, 229, 84–93. [Google Scholar] [CrossRef]
- Maaroff, R.M.; Md Jahim, J.; Azahar, A.M.; Abdul, P.M.; Masdar, M.S.; Nordin, D.; Abd Nasir, M.A. Biohydrogen production from palm oil mill effluent (POME) by two stage anaerobic sequencing batch reactor (ASBR) system for better utilization of carbon sources in POME. Int. J. Hydrogen Energy 2019, 3395–3406. [Google Scholar] [CrossRef]
- Zainal, B.S.; Danaee, M.; Mohd, N.S.; Ibrahim, S. Effects of temperature and dark fermentation effluent on biomethane production in a two-stage up-flow anaerobic sludge fixed-film (UASFF) bioreactor. Fuel 2020, 263, 116729. [Google Scholar] [CrossRef]
- Mishra, P.; Thakur, S.; Singh, L.; Wahid, Z.A.; Sakinah, M.; Ab Wahid, Z.; Sakinah, M. Enhanced hydrogen production from palm oil mill effluent using two stage sequential dark and photo fermentation. Int. J. Hydrogen Energy 2016, 41, 18431–18440. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.F.; Li, Y.Y.; Xu, K.Q.; Ebie, Y.; Inamori, Y.; Kong, H.N. A pH- and temperature-phased two-stage process for hydrogen and methane production from food waste. Int. J. Hydrogen Energy 2008, 33, 4739–4746. [Google Scholar] [CrossRef]
- Zahedi, S.; Solera, R.; Micolucci, F.; Cavinato, C.; Bolzonella, D. Changes in microbial community during hydrogen and methane production in two-stage thermophilic anaerobic co-digestion process from biowaste. Waste Manag. 2016, 49, 40–46. [Google Scholar] [CrossRef]
- Ueno, Y.; Fukui, H.; Goto, M. Operation of a two-stage fermentation process producing hydrogen and methane from organic waste. Environ. Sci. Technol. 2007, 41, 1413–1419. [Google Scholar] [CrossRef]
- Lee, D.; Ebie, Y.; Xu, K.; Li, Y.; Inamori, Y. Continuous H2 and CH4 production from high-solid food waste in the two-stage thermophilic fermentation process with the recirculation of digester sludge. Bioresour. Technol. 2010, 101, S42–S47. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Stamatelatou, K.; Venetsaneas, N.; Kornaros, M.; Lyberatos, G. Biohydrogen and methane production from cheese whey in a two-stage anaerobic process. Ind. Eng. Chem. Res. 2008, 47, 5227–5233. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, D.H.; Lee, M.Y.; Shin, H.S. Two-stage UASB reactor converting coffee drink manufacturing wastewater to hydrogen and methane. Int. J. Hydrogen Energy 2012, 37, 7473–7481. [Google Scholar] [CrossRef]
- Angeriz-Campoy, R.; Álvarez-Gallego, C.J.; Romero-García, L.I. Thermophilic anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW) with food waste (FW): Enhancement of bio-hydrogen production. Bioresour. Technol. 2015, 194, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen production from biomass and wastes via dark fermentation: A review. Waste Biomass Valorization 2010, 1, 21–39. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrre, H.; Steyer, J.P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Piera, M.; Martínez-Val, J.M.; José Montes, M. Safety issues of nuclear production of hydrogen. Energy Convers. Manag. 2006, 47, 2732–2739. [Google Scholar] [CrossRef]
- Redwood, M.D.; Paterson-Beedle, M.; MacAskie, L.E. Integrating dark and light bio-hydrogen production strategies: Towards the hydrogen economy. Rev. Environ. Sci. Biotechnol. 2009, 8, 149–185. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Krishna, S. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Ramachandran, R.A.M. An overview of industrial uses of hydrogen. Int. J. Hydrogen Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Lattin, W.C.; Utgikar, V.P. Transition to hydrogen economy in the United States: A 2006 status report. Int. J. Hydrogen Energy 2007, 32, 3230–3237. [Google Scholar] [CrossRef]
- Mueller-langer, F.; Tzimas, E.; Kaltschmitt, M.; Peteves, S. Techno-economic assessment of hydrogen production processes for the hydrogen economy for the short and medium term. Int. J. Hydrogen Energy 2007, 32, 3797–3810. [Google Scholar] [CrossRef]
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrogen Energy 2003, 28, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Eliezer, D.; Eliaz, N.; Senkov, O.N.; Froes, F.H. Positive effects of hydrogen in metals. Mater. Sci. Eng. A 2000, 280, 220–224. [Google Scholar] [CrossRef]
- Eliaz, N.; Eliezer, D.; Olson, D.L. Hydrogen-assisted processing of materials. Mater. Sci. Eng. A 2000, 289, 41–53. [Google Scholar] [CrossRef]
- Dupont, V. Steam reforming of sunflower oil for hydrogen gas production. HELIA 2007, 30, 103–132. [Google Scholar]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the fuel for 21st century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Pagliaccia, P.; Gallipoli, A.; Gianico, A.; Montecchio, D.; Braguglia, C.M. Single stage anaerobic bioconversion of food waste in mono and co-digestion with olive husks: Impact of thermal pretreatment on hydrogen and methane production. Int. J. Hydrogen Energy 2016, 41, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Chynoweth, D.P. Renewable Biomethane From Land and Ocean Energy Crops and Organic Wastes. Hortic. Sci. 2005, 40, 283–286. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Unit | Raw POME | Digested POME | References |
|---|---|---|---|---|
| Mean ± S.D. * | Mean ± S.D. * | |||
| pH | – | 4.3 ± 0.28 | 7.4 ± 0.05 | [15,16,17,18] |
| Volatile fatty acids (VFAs) | mg L−1 | 470 ± 240 | 678.83 ± 166.47 | [15,19,20] |
| Chemical oxygen demand (COD) | mg L−1 | 53,450 ± 10,350 | 83,800 ± 11,000 | [16,21,22,23] |
| Total suspended solids (TSS) | mg L−1 | 29,000 ± 6000 | 10,200 ± 2500 | [22,23,24] |
| Suspended solids (SS) | mg L−1 | 23,600 ± 4400 | 4126 | [22,25] |
| Oil and grease | mg L−1 | 7000 ± 550 | 183 ± 10.1 | [21,22,26] |
| Ammonium nitrogen (NH3–N) | mg L−1 | 63 ± 24 | 25 ± 5 | [22,25,23] |
| Biochemical oxygen demand (BOD) | mg L−1 | 28,000 ± 6750 | 19,000 ± 5500 | [21,22,26,27] |
| Parameter | Limits Required Based on the Period of Discharge | ||||||
|---|---|---|---|---|---|---|---|
| 1 July 1978–30 June 1979 | 1 July 1979–30 June 1980 | 1 July 1980–30 June 1981 | 1 July 1981–30 June 1982 | 1 July 1982–31 December 1983 | 1 January 1984–2015 | Future Standard Discharge Limit (2015 Onwards) | |
| pH | 5–9 | 5–9 | 5–9 | 5–9 | 5–9 | 5–9 | 5–9 |
| Temperature (°C) | 45 | 45 | 45 | 45 | 45 | 45 | 45 |
| Oil and Grease (mg L−1) | 150 | 100 | 75 | 50 | 50 | 50 | 5 |
| Total Solids (mg L−1) | 4000 | 2500 | 2000 | 1500 | - | - | - |
| Suspended Solids (mg L−1) | 1200 | 800 | 600 | 400 | 400 | 400 | 200 |
| Total Nitrogen (mg L−1) | 200 | 100 | 75 | 50 | - | - | 150 |
| Ammonium Nitrogen (mg L−1) | 25 | 15 | 15 | 10 | 150 | 100 | - |
| COD (mg L−1) | 10,000 | 4000 | 2000 | 1000 | 100 | 100 | - |
| BOD (mg L−1) | 5000 | 2000 | 1000 | 500 | 250 | 100 | 20 |
| Treatment Method Used | Parameters | References | ||
|---|---|---|---|---|
| BOD removal (%) | COD removal (%) | Total suspended solids/suspended solids removal (%) | ||
| Lab scale | ||||
| Using biosorbent | 97.41 | 100 | 100 | [42] |
| Ultrasonic-assisted membrane anaerobic system | 74 | 95 | 91–99.5 | [43] |
| Attached growth on rotating biological contactor | 91 | 88 | 89 | [44] |
| Large scale | ||||
| Anaerobic expanded granular sludge bed (EGSB) | 88.24 | 94.89 | 64.65 | [45] |
| Combined high-rate anaerobic reactors | - | 93.50 | >90 | [46] |
| Ultrafiltration membrane | 86.33 | 85 | 99.86 | [47] |
| Activated carbon as bioadsorbent | 83 | 68 | 90 | [48] |
| Green synthesis | - | 94.70 | 51.50 | [49] |
| Inoculum | Bioreactor | Organic Loading Rate (OLR; g L−1 d−1) | Temperature (°C) | HPR (L H2 L−1 d−1) | MPR (L CH4 L−1 d−1) | COD Removal (%) | References |
|---|---|---|---|---|---|---|---|
| Digested POME | 500 mL serum bottle | 4.96 | 37 | 5.99 ± 0.5 | - | 42 | [65] |
| Digested POME | UASFF | 9.43 | 50 | - | 4.40 | 94 | [17] |
| Digested POME | CSTR | 25 | 55 | 1.16 | - | <30 | [88] |
| Digested POME | UASFF | 51.8 | 38 | 4.61 | - | 40–54 | [89] |
| Digested POME | 50-L UASB | 500–1000 | 30–35 | - | 992 | >90 | [54] |
| Inoculum | Integrated System Used | Organic Loading Rate (OLR; g L−1 d−1) H2 CH4 | Temperature (°C) H2 CH4 | HPR (L H2 L−1 d−1) | MPR (L CH4 L−1 d−1) | COD Removal (%) H2 CH4 | References |
|---|---|---|---|---|---|---|---|
| Anaerobic seed sludge | DF–AD (UASB–CSTR) | 75 30 | 55 37 | 1.92 | 3.20 | 42 94 | [90] |
| Decanter cake | DF–AD (two-stage batch fermentation system) | 60 g VS L−1d−1 60 g VS L−1d−1 | 60 60 | 1.46 a | 51.59 a | - | [91] |
| POME sludge | DF–AD (UASFF–UASFF) | 20 varies | 43 43 | 5.29 | 9.60 | 26 79 | [18] |
| POME sludge | DF–AD (ASBR–UASB) | 60 6 | 55 35 | 1.804 | 2.60 | 38 95 | [92] |
| POME sludge | DF–AD (CSTR–UASB) | 14.3 g VS L−1d−1 1.58 g VS L−1d−1 | 55 35 | 3.80 | 14.00 | 93 | [93] |
| Anaerobic Treatment System | Advantages | Disadvantages | References |
|---|---|---|---|
| UASB | High COD removal efficiency and CH4 production rate | High dependable on sludge settling property | [41] |
| UASFF | Higher biomass retention, a shorter start-up for sludge granulation | Reactor stability and efficiency depend on the feed flow rate, internal packing, up-flow velocity and effluent recycle ratio | [94] |
| ASBR | Simple operation, flexible and no separate clarifiers needed. | Low treatment capability under higher OLR | [73] |
| CSTR | Inexpensive and easy to handle | Poor gas production under high OLR and short HRT | [95] |
| Types of Waste | Inoculum | Integrated System Applied | Organic Loading Rate (OLR; g L−1 d−1) H2 CH4 | Temperature (°C) H2 CH4 | HPR (L H2 L−1 d−1) | MPR (L CH4 L−1 d−1) | COD Removal (%) H2 CH4 | References |
|---|---|---|---|---|---|---|---|---|
| Organic Fraction of Municipal Solid Waste | Waste activated sludge | DF–AD (CSTR–CSTR) | 16 kg TVS m3 −1 d−1 3 kg TVS m3 −1 d−1 | 55 55 | 0.43 ± 0.04 | 0.60 ± 0.09 | 43 52) | [105] |
| Garbage slurry and shredded office papers | Seed microflora | DF–AD (CSTR–Packed Bed Reactor) | 97,000 15,700 | 60 55 | 5400 | 6100 | - 79 | [106] |
| Food waste from organic fraction municipal solid wastes (OFMSW) | Anaerobic digester sludge | DF–AD (Semi-continuous mode) | 39 4.16 | 55 55 | 11.1 | 47.4 | 90 85 | [107] |
| Sugarcane syrup | Brewery UASB granules | DF–AD (CSTR–ABR*) | 2167 10,773 | 35 35 | 7.53 | 75.6 | 69 94 | [108] |
| Coffee drink manufacturing wastewater (CDMW) | Anaerobic seed sludge | DF–AD (UASB–UASB) | 80 3.5 | 55 35 | 101.76 | 2.06 | 50 93 | [109] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainal, B.S.; Ahmad, M.A.; Danaee, M.; Jamadon, N.; Mohd, N.S.; Ibrahim, S. Integrated System Technology of POME Treatment for Biohydrogen and Biomethane Production in Malaysia. Appl. Sci. 2020, 10, 951. https://doi.org/10.3390/app10030951
Zainal BS, Ahmad MA, Danaee M, Jamadon N, Mohd NS, Ibrahim S. Integrated System Technology of POME Treatment for Biohydrogen and Biomethane Production in Malaysia. Applied Sciences. 2020; 10(3):951. https://doi.org/10.3390/app10030951
Chicago/Turabian StyleZainal, Bidattul Syirat, Mohd Azwan Ahmad, Mahmoud Danaee, Nashrah Jamadon, Nuruol Syuhadaa Mohd, and Shaliza Ibrahim. 2020. "Integrated System Technology of POME Treatment for Biohydrogen and Biomethane Production in Malaysia" Applied Sciences 10, no. 3: 951. https://doi.org/10.3390/app10030951
APA StyleZainal, B. S., Ahmad, M. A., Danaee, M., Jamadon, N., Mohd, N. S., & Ibrahim, S. (2020). Integrated System Technology of POME Treatment for Biohydrogen and Biomethane Production in Malaysia. Applied Sciences, 10(3), 951. https://doi.org/10.3390/app10030951