Antifungal Properties of Pure Silver Films with Nanoparticles Induced by Pulsed-Laser Dewetting Process
Abstract
:1. Introduction
2. Experiment
2.1. Silver Particle Preparation
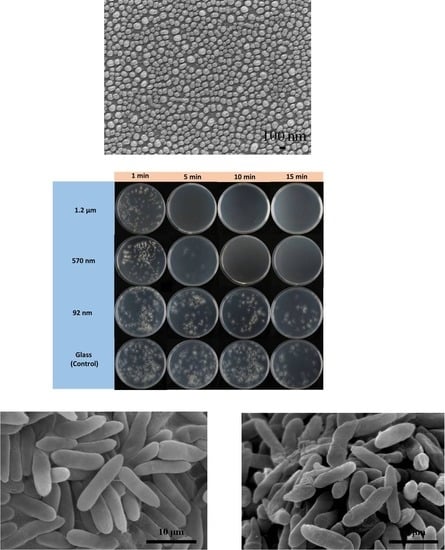
2.2. Antifungal Test
2.3. Spore germination Inhibition Assay
2.4. Morphology Analysis
2.5. Virulence Assay
2.6. RT-PCR Analysis of Gene Expression
2.7. Morphology of C. Gloeosporioides Conidia
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khalid Akhtar, P.; Alam, S.S. Assessment keys for some important diseases of mango. Pak. J. Biol. Sci. 2002, 5, 246–250. [Google Scholar]
- Tucho, A.; Lemessa, F.; Berecha, G. Distribution and occurrence of mango anthracnose (colletotrichum gloesporioides penz and sacc) in humid agro-ecology of southwest ethiopia. Plant Pathol. J. 2014, 13, 268–277. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Villapún, V.; Dover, L.; Cross, A.; González, S. Antibacterial Metallic Touch Surfaces. Materials 2016, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Huang, Y.S.; Lin, Y.S.; Lin, C.H.; Huang, J.C.; Chen, C.H.; Li, J.B.; Chen, Y.H.; Jang, J.S.C. Electrochemical and biocompatibility response of newly developed TiZr-based metallic glasses. Mater. Sci. Eng. C 2014, 43, 343–349. [Google Scholar] [CrossRef]
- Mahdizadeh, V.; Safaie, N.; Khelghatibana, F. Evaluation of antifungal activity of silver nanoparticles against some phytopathogenic fungi and Trichoderma harzianum. J. Crop Prot. 2015, 4, 291–300. [Google Scholar]
- Herman, A.; Herman, A.P. Nanoparticles as Antimicrobial Agents: Their Toxicity and Mechanisms of Action. J. Nanosci. Nanotechnol. 2014, 14, 946–957. [Google Scholar] [CrossRef]
- Soo-Hwan, K.; Hyeong-Seon, L.; Deok-Seon, R.; Soo-Jae, C.; Lee, D.-S. Antibacterial Activity of Silver-nanoparticles Against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Nie, C.; Yang, Y.; Cheng, C.; Ma, L.; Deng, J.; Wang, L.; Zhao, C. Bioinspired and biocompatible carbon nanotube-Ag nanohybrid coatings for robust antibacterial applications. Acta Biomater. 2017, 51, 479–494. [Google Scholar] [CrossRef]
- Chowdappa, P.; Gowda, S.; Chethana, S.C.; Madhura, S. Antifungal activity of chitosan-silver nanoparticle composite against Colletotrichum gloeosporioides associated with mango anthracnose. Afr. J. Microbiol. Res. 2014, 8, 1803–1812. [Google Scholar] [CrossRef] [Green Version]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.P.; Jang, J.S.C.; Huang, J.C.; Chou, H.S.; Yang, Y.; Ye, J.C.; Wang, Y.C.; Lee, J.W.; Liu, F.X.; Liaw, P.K.; et al. Thin film metallic glasses: Unique properties and potential applications. Thin Solid Film. 2012, 520, 5097–5122. [Google Scholar] [CrossRef]
- Huang, J.C.; Chu, J.P.; Jang, J.S.C. Recent progress in metallic glasses in Taiwan. Intermetallics 2009, 17, 973–987. [Google Scholar] [CrossRef]
- Chen, H.-W.; Hsu, K.-C.; Chan, Y.-C.; Duh, J.-G.; Lee, J.-W.; Jang, J.S.-C.; Chen, G.-J. Antimicrobial properties of Zr–Cu–Al–Ag thin film metallic glass. Thin Solid Film. 2014, 561, 98–101. [Google Scholar] [CrossRef]
- Kanhed, P.; Birla, S.; Gaikwad, S.; Gade, A.; Seabra, A.B.; Rubilar, O.; Duran, N.; Rai, M. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Janbua, W.; Bongkarn, T.; Vittayakorn, W.; Vittayakorn, N. Direct synthesis and growth mechanism of metal molybdate (AMoO 4; A = Ca and Ba) fine particles via the mechanochemical method. Ceram. Int. 2017, 43, S435–S443. [Google Scholar] [CrossRef]
- Velgosová, O.; Mražíková, A.; Marcinčáková, R. Influence of pH on green synthesis of Ag nanoparticles. Mater. Lett. 2016, 180, 336–339. [Google Scholar] [CrossRef]
- Kumar, N.; Alam, F.; Dutta, V. Deposition of Ag and Au–Ag alloy nanoparticle films by spray pyrolysis technique with tuned plasmonic properties. J. Alloy. Compd. 2014, 585, 312–317. [Google Scholar] [CrossRef]
- Raffi, M.; Akhter, J.I.; Hasan, M.M. Effect of annealing temperature on Ag nano-composite synthesized by sol–gel. Mater. Chem. Phys. 2006, 99, 405–409. [Google Scholar] [CrossRef]
- Bradley, J.-C.; Babu, S.; Carroll, B.; Mittal, A. A study of spatially coupled bipolar electrochemistry on the sub-micrometer scale: Colloidal particles on surfaces and cylinders in nuclear-track etched membranes. J. Electroanal. Chem. 2002, 522, 75–85. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, M. Single-pulse transformation of Ag thin film into nanoparticles via laser-induced dewetting. Appl. Surf. Sci. 2017, 399, 555–564. [Google Scholar] [CrossRef]
- Kumar, K.; Swaminathan, P. Role of silver nanoparticles in the dewetting behavior of copper thin films. Thin Solid Film. 2017, 642, 364–369. [Google Scholar] [CrossRef]
- Datta, D.P.; Chettah, A.; Siva, V.; Kanjilal, D.; Sahoo, P.K. Dewetting induced Au-Ge composite nanodot evolution in SiO2. Appl. Surf. Sci. 2018, 428, 676–683. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, J.; Lee, M. Fabrication of Ag-Au bimetallic nanoparticles by laser-induced dewetting of bilayer films. Appl. Surf. Sci. 2018, 434, 1293–1299. [Google Scholar] [CrossRef]
- Maurya, S.K.; Uto, Y.; Kashihara, K.; Yonekura, N.; Nakajima, T. Rapid formation of nanostructures in Au films using a CO 2 laser. Appl. Surf. Sci. 2018, 427, 961–965. [Google Scholar] [CrossRef]
- Heinz, M.; Srabionyan, V.V.; Avakyan, L.A.; Bugaev, A.L.; Skidanenko, A.V.; Kaptelinin, S.Y.; Ihlemann, J.; Meinertz, J.; Patzig, C.; Dubiel, M.; et al. Formation of bimetallic gold-silver nanoparticles in glass by UV laser irradiation. J. Alloy. Compd. 2018, 767, 1253–1263. [Google Scholar] [CrossRef]
- Kline, J.E.; Leonard, J.P. Suppression of dewetting phenomena during excimer laser melting of thin metal films on SiO2. Thin Solid Film. 2005, 488, 306–313. [Google Scholar] [CrossRef]
- Makarov, S.V.; Milichko, V.A.; Mukhin, I.S.; Shishkin, I.I.; Zuev, D.A.; Mozharov, A.M.; Krasnok, A.E.; Belov, P.A. Controllable femtosecond laser-induced dewetting for plasmonic applications. Laser Photonics Rev. 2016, 10, 91–99. [Google Scholar] [CrossRef]
- Busleev, N.I.; Ivanova, A.K.; Kudryashov, S.I.; Rudenko, A.A.; Zayarny, D.A.; Ionin, A.A. Fano Resonances as Optical Markers of Sub-Wavelength Nanoparticle Packaging and Elemental Segregation in Laser-Dewetted Au-Pd Film. Plasmonics 2019, 14, 2013–2019. [Google Scholar] [CrossRef]
- Nastulyavichus, A.A.; Smirnov, N.A.; Kudryashov, S.I.; Ionin, A.A.; Saraeva, I.N.; Busleev, N.I.; Rudenko, A.A.; Khmel’nitskii, R.A.; Zayarnyi, D.A. Formation of nanoparticles from thin silver films irradiated by laser pulses in air. Quantum Electron. 2018, 48, 251–254. [Google Scholar] [CrossRef]
- Benware, B.R.; Macchietto, C.D.; Moreno, C.H.; Rocca, J.J. Demonstration of a high average power tabletop soft X-Ray Laser. Phys. Rev. Lett. 1998, 81, 5804–5807. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Pu, J.; Zhang, H.; Liu, Y.; Zhou, F.; Zhang, K.; Liu, X. The laccase gene (LAC1) is essential for Colletotrichum gloeosporioides development and virulence on mango leaves and fruits. Physiol. Mol. Plant Pathol. 2017, 99, 55–64. [Google Scholar] [CrossRef]
- Leroy, F.; Borowik, Ł.; Cheynis, F.; Almadori, Y.; Curiotto, S.; Trautmann, M.; Barbé, J.C.; Müller, P. How to control solid state dewetting: A short review. Surf. Sci. Rep. 2016, 71, 391–409. [Google Scholar] [CrossRef]
- Gazit, N.; Klinger, L.; Rabkin, E. Chemically-induced solid-state dewetting of thin Au films. Acta Mater. 2017, 129, 300–311. [Google Scholar] [CrossRef]
- Lo Savio, R.; Repetto, L.; Guida, P.; Angeli, E.; Firpo, G.; Volpe, A.; Ierardi, V.; Valbusa, U. Control of the micrometric scale morphology of silicon nanowires through ion irradiation-induced metal dewetting. Solid State Commun. 2016, 240, 41–45. [Google Scholar] [CrossRef]
- Ouda, S.M. Antifungal activity of sliver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2014, 9, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.Y.; Lin, Y.S.; Chang, C.M.; Liu, J.K.; Chen, C.H.; Huang, J.C. Promising antimicrobial capability of thin film metallic glasses. Mater. Sci. Eng. CMater. Biol. Appl. 2014, 36, 221–225. [Google Scholar] [CrossRef]
- Ashajyothi, C.; Prabhurajeshwar, C.; Handral, H.K.; Kelmani, R.C. Investigation of antifungal and anti-mycelium activities using biogenic nanoparticles: An eco-friendly approach. Environ. Nanotechnol. Monit. Manag. 2016, 5, 81–87. [Google Scholar] [CrossRef]










| Sample Particle Size | Film Thickness (nm) | Roughness (nm) | Contact Angle (°) |
|---|---|---|---|
| Glass | - | 0.3 | 63 ± 2 |
| 92 nm | 10 | 5 ± 2 | 43 ± 2 |
| 570 nm | 50 | 44 ± 1 | 51 ± 2 |
| 1.2 µm | 50 | 73 ± 3 | 73 ± 1 |
| Treatment | Lesion Size (mm) | |
|---|---|---|
| Wound Made | Non-Wound Made | |
| Control (Glass) | 15.56 ± 0.76 a | 13.10 ± 0.91 a |
| 92 nm | 13.50 ± 0.66 b | 7.00 ± 1.95 b |
| 570 nm | 11.75 ± 0.69 c | 6.80 ± 1.42 b |
| 1.2 µm | 11.00 ± 0.66 c | 3.30 ± 1.15 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Wang, J.-J.; Wang, Y.-T.; Lin, H.-K.; Lin, Y.-J. Antifungal Properties of Pure Silver Films with Nanoparticles Induced by Pulsed-Laser Dewetting Process. Appl. Sci. 2020, 10, 2260. https://doi.org/10.3390/app10072260
Lin Y-H, Wang J-J, Wang Y-T, Lin H-K, Lin Y-J. Antifungal Properties of Pure Silver Films with Nanoparticles Induced by Pulsed-Laser Dewetting Process. Applied Sciences. 2020; 10(7):2260. https://doi.org/10.3390/app10072260
Chicago/Turabian StyleLin, Ying-Hong, Jyun-Jhih Wang, Yung-Ting Wang, Hsuan-Kai Lin, and Yi-Jia Lin. 2020. "Antifungal Properties of Pure Silver Films with Nanoparticles Induced by Pulsed-Laser Dewetting Process" Applied Sciences 10, no. 7: 2260. https://doi.org/10.3390/app10072260
APA StyleLin, Y. -H., Wang, J. -J., Wang, Y. -T., Lin, H. -K., & Lin, Y. -J. (2020). Antifungal Properties of Pure Silver Films with Nanoparticles Induced by Pulsed-Laser Dewetting Process. Applied Sciences, 10(7), 2260. https://doi.org/10.3390/app10072260