Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation
Abstract
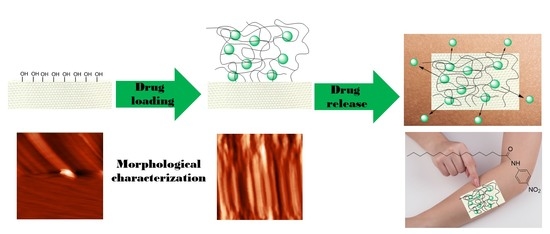
:1. Introduction
2. Materials and Methods
3. Results
3.1. Sol–Gel Synthesis and Coating Application
3.2. Sol NMR Characterization
3.3. ATR FT-IR
3.4. Morphological Characterizations
3.5. AFM Characterizations
3.6. PNPA in Vitro Diffusion Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar]
- Brouwers, J.R.B.J. Advanced and controlled drug delivery systems in clinical disease management. Pharm. World Sci. 1996, 18, 153–162. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Rosano, C.; Randino, R.; Caruso, A.; Saturnino, C.; Muià, N.; Ceramella, J.; Puoci, F.; Rodriquez, M.; et al. N-thioalkylcarbazoles derivatives as new anti-proliferative agents: Synthesis, characterisation and molecular mechanism evaluation. J. Enzyme Inhib. Med. Chem. 2018, 33, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Saturnino, C.; Caruso, A.; Longo, P.; Capasso, A.; Pingitore, A.; Caroleo, M.C.; Cione, E.; Perri, M.; Nicolò, F.; Nardo, V.M.; et al. Crystallographic study and biological evaluation of 1,4-dimethyl-N-alkylcarbazoles. Curr. Top. Med. Chem. 2015, 15, 973–979. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef]
- Brannon-Peppas, L. Controlled Release in the Food and Cosmetics Industries. In Polymeric Delivery Systems—Chapter 3; El-Nokaly, M.A., Piatt, D.M., Charpentier, B.A., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 42–52. [Google Scholar]
- Levy, R.; Nichols, M.A.; Miller, T.W., Jr. Encapsulated Systems for Controlled Release and Pest Management. In Polymeric Delivery Systems—Chapter 13; El-Nokaly, M.A., Piatt, D.M., Charpentier, B.A., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 202–212. [Google Scholar]
- Radu, C.D.; Parteni, O.; Popa, M.; Muresan, I.E.; Ochiuz, L.; Bulgariu, L.; Munteanu, C.; Istrate, B.; Ulea, E. Comparative study of a drug release from a textile to skin. J. Pharm. Drug Deliv. Res. 2015, 4. [Google Scholar] [CrossRef]
- Hashemikia, S.; Hemmatinejad, N.; Ahmadi, E.; Montazer, M. A novel cotton fabric with anti-bacterial and drug delivery properties using SBA-15-NH 2/polysiloxane hybrid containing tetracycline. Mater. Sci. Eng. C Mater. 2016, 59, 429–437. [Google Scholar] [CrossRef]
- Ten Breteler, M.R.; Nierstrasz, V.A.; Warmoeskerken, M.M.C.G. Textile slow-release systems with medical applications. Autex Res. J. 2002, 2, 175–189. [Google Scholar]
- Radu, C.D.; Popa, M.; Parteni, O.; Salariu, M.; Lupușoru, E.C.; Ghiciuc, C.; Foia, L.; Chiriac, A.; Lupusoru, R.; Oproiu, L.; et al. Achievements and limits on the controlled release of a drug from a textile fabric to dermis. Open Conf. Proc. J. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Radu, C.D.; Parteni, O.; Ochiuz, L. Applications of cyclodextrins in medical textiles—Review. J. Control Release 2016, 224, 146–157. [Google Scholar] [CrossRef]
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol-gel coatings. J. Mater. Chem. 2005, 15, 4385. [Google Scholar] [CrossRef]
- Plutino, M.R.; Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Maschi, O.; Mezzi, A.; Rosace, G. Sol-gel 3-glycidoxypropyltriethoxysilane finishing on different fabrics: The role of precursor concentration and catalyst on the textile performances and cytotoxic activity. J. Colloid Interf. Sci. 2017, 506, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Development of a textile-optoelectronic pH meter based on hybrid xerogel doped with methyl red. Sens. Actuators B Chem. 2012, 171–172, 1013–1021. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K.; Brancatelli, G.; Rosace, G.; Van Damme, E.; De Vos, W. Novel cellulose and polyamide halochromic textile sensors based on the encapsulation of Methyl Red into a sol-gel matrix. Sens. Actuators B Chem. 2012, 162, 27–34. [Google Scholar] [CrossRef]
- Guido, E.; Colleoni, C.; De Clerck, K.; Plutino, M.R.; Rosace, G. Influence of catalyst in the synthesis of a cellulose-based sensor: Kinetic study of 3-glycidoxypropyltrimethoxysilane epoxy ring opening by Lewis acid. Sens. Actuators B Chem. 2014, 203, 213–222. [Google Scholar] [CrossRef]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens. Actuators B Chem. 2016, 222, 213–220. [Google Scholar] [CrossRef]
- Plutino, M.R.; Guido, E.; Colleoni, C.; Rosace, G. Effect of GPTMS functionalization on the improvement of the pH-sensitive methyl red photostability. Sens. Actuators B Chem. 2017, 238, 281–291. [Google Scholar] [CrossRef]
- Rosace, G.; Guido, E.; Colleoni, C.; Brucale, M.; Piperopoulos, E.; Milone, C.; Plutino, M.R. Halochromic resorufin-GPTMS hybrid sol-gel: Chemical-physical properties and use as pH sensor fabric coating. Sens. Actuators B Chem. 2017, 241, 85–95. [Google Scholar] [CrossRef]
- Trovato, V.; Colleoni, C.; Castellano, A.; Plutino, M.R. The key role of 3-glycidoxypropyltrimethoxysilane sol-gel precursor in the development of wearable sensors for health monitoring. J. Sol-Gel Sci. Technol. 2018, 87, 27–40. [Google Scholar] [CrossRef]
- Poli, R.; Colleoni, C.; Calvimontes, A.; Polášková, H.; Dutschk, V.; Rosace, R. Innovative sol-gel route in neutral hydroalcoholic condition to obtain antibacterial cotton finishing by zinc precursor. J. Sol-Gel Sci. Technol. 2015, 74, 151–160. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent bio-applications of sol-gel materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Cardiano, P. Hydrophobic properties of new epoxy-silica hybrids. J. Appl. Polym. Sci. 2008, 108, 3380–3387. [Google Scholar] [CrossRef]
- Cardiano, P.; Lo Schiavo, S.; Piraino, P. Hydrorepellent properties of organic–inorganic hybrid materials. J. Non-Cryst. Solids 2010, 356, 917–926. [Google Scholar] [CrossRef]
- Brancatelli, G.; Colleoni, C.; Massafra, M.R.; Rosace, G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym. Degrad. Stabil. 2011, 96, 483–490. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Colleoni, C.; Guido, E.; Botteri, L.; Rosace, G. Thermal behaviour and flame retardancy of monoethanolamine-doped sol-gel coatings of cotton fabric. Prog. Org. Coat. 2017, 103, 174–181. [Google Scholar] [CrossRef]
- Saturnino, C.; Popolo, A.; Ramunno, A.; Adesso, S.; Pecoraro, M.; Plutino, M.R.; Rizzato, S.; Albinati, A.; Marzocco, S.; Sala, M.; et al. Anti-inflammatory, antioxidant and crystallographic studies of N-Palmitoyl-ethanol Amine (PEA) derivatives. Molecules 2017, 22, 616. [Google Scholar] [CrossRef] [Green Version]
- Parisi, O.; Scrivano, L.; Amone, F.; Malivindi, R.; Ruffo, M.; Vattimo, A.F.; Pezzi, V.; Puoci, F. Interconnected PolymerS TeChnology (IPSTiC): An effective approach for the modulation of 5α-reductase activity in hair loss conditions. J. Funct. Biomater. 2018, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stabil. 2019, 162, 148–159. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohyd. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Peng, Y.D.; Wang, F.; Gao, L.; Dong, W.K. Structurally characterized dinuclear Zinc(II) bis(salamo)-type tetraoxime complex possessing square pyramidal and trigonal bipyramidal geometries. J. Chin. Chem. Soc. Taipei 2018, 65, 893–899. [Google Scholar] [CrossRef]
- Ma, Z.H.; Yu, D.G.; Branford-White, C.J.; Nie, H.L.; Fan, Z.X.; Zhu, L.M. Microencapsulation of tamoxifen: Application to cotton fabric. Colloid Surf. B 2009, 69, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Messaritaki, A.; Black, S.J.; van der Walle, C.F.; Rigby, S.P. NMR and confocal microscopy studies of the mechanisms of burst drug release from PLGA microspheres. J. Control Release 2005, 108, 271–281. [Google Scholar] [CrossRef] [PubMed]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Appl. Sci. 2020, 10, 2287. https://doi.org/10.3390/app10072287
Puoci F, Saturnino C, Trovato V, Iacopetta D, Piperopoulos E, Triolo C, Bonomo MG, Drommi D, Parisi OI, Milone C, et al. Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Applied Sciences. 2020; 10(7):2287. https://doi.org/10.3390/app10072287
Chicago/Turabian StylePuoci, Francesco, Carmela Saturnino, Valentina Trovato, Domenico Iacopetta, Elpida Piperopoulos, Claudia Triolo, Maria Grazia Bonomo, Dario Drommi, Ortensia Ilaria Parisi, Candida Milone, and et al. 2020. "Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation" Applied Sciences 10, no. 7: 2287. https://doi.org/10.3390/app10072287
APA StylePuoci, F., Saturnino, C., Trovato, V., Iacopetta, D., Piperopoulos, E., Triolo, C., Bonomo, M. G., Drommi, D., Parisi, O. I., Milone, C., Sinicropi, M. S., Rosace, G., & Plutino, M. R. (2020). Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Applied Sciences, 10(7), 2287. https://doi.org/10.3390/app10072287